The Sheer Scale of the Problem
While dementia is usually regarded as a disorder of old age, it can invariably also affect younger individuals under the age of 651. Early-onset dementia (EOD), also known as young-onset dementia (YOD), is defined as dementia with symptom onset before the age of 65 (or under the age of 45 as in the case of YOD) and carries a significant burden on patients and their families2. The prevalence has been variably reported to range between 38 and 420 EOD cases per 100,000 in the 30 to 64 age group2. Patients with EOD are not only often diagnosed much later than late-onset dementia as they are not referred to specialists in a timely manner, but the diagnosis is also often challenging2. Although there is more awareness of reducing risk factors such as smoking and alcohol consumption in developed countries, such a trend is rarely seen in developing countries, and the disease burden of EOD is increasing substantially3. We aim to highlight the challenges of EOD faced by patients and healthcare professionals and, in addition, to discuss the recent breakthrough in this area.
The Different Faces of EOD
Patients with EOD often present with atypical symptoms that are not commonly recognised as a part of dementia2. Moreover, the genetic or idiopathic aetiology of EOD makes it even more difficult to diagnose, as literature has shown that individuals with EOD may go through 4.7 arduous years of referrals after seeing two to five consultants, before finally receiving an accurate diagnosis4. For instance, individuals with frontotemporal dementia (FTD) may display inappropriate behaviours, significant apathy, personality changes, or speech difficulties5. 40% of FTDs are misdiagnosed as Alzheimer’s disease (AD), psychiatric disorders, or Parkinson’s disease (PD), despite having a completely different pathophysiology that instead may overlap with other neurological diseases, such as motor neuron disease, amyotrophic lateral sclerosis (ALS), progressive supranuclear palsy syndrome, and corticobasal syndrome5,6. This may then affect the treatment outcomes in these patients as cholinesterase inhibitors and memantine are frequently prescribed in patients with AD, but are deemed ineffective in patients with FTD7. In addition, FTD is a common type of EOD in Hong Kong with a high genetic predisposition8, making the diagnosis of the condition clinically challenging for both patients and clinicians.
The situation is similar for Lewy body dementia (LBD) that has overlapping symptoms with PD since the intraneuronal α-synuclein aggregation is seen in both conditions9. Consequently, though LBD is not as common in Hong Kong as in other populations, it is more likely that the unique features of LBD (fluctuating levels of cognitive impairment, visual hallucinations and a history of sleep disturbances related to rapid eye movement sleep behaviour disorder) are not pieced together in patients who are then misdiagnosed with LBD’s parkinsonism counterpart9, 10. Recent genome-wide association studies have confirmed that the infamous apolipoprotein-E4 (ApoE4) variant associated with familial AD also increases the risk of developing LBD11. Approximately 50% of local cases of AD often have coexisting LBD or Parkinson’s disease dementia (PDD)9, and the diagnosis of LBD may be missed, thereby affecting the treatment outcomes in such patients. Notably, a correct diagnosis of LBD or PDD in patients carries an important bearing on the subsequent treatment since neuroleptic drugs in these patients should be avoided, owing to the high risk of neuroleptic syndrome seen in LBD/PDD10. Without sufficient awareness of the disease plagued by the late diagnoses, patients are unable to access appropriate treatment and management strategies, and may exacerbate the disease progression and instil psychological effects of the disease burden12.
Moving Beyond Memory and the Burden of Care
EOD further shift the burden of care provided to Hong Kong’s ageing population, with additional challenges such as having one of the world’s highest life expectancies, a delayed retirement age and an overstretched healthcare system12. Furthermore, with a mean age of EOD onset at 58 (the youngest local patient was aged 38 in Hong Kong), individuals in the working class are often left in a dilemma when they experience the atypical symptoms of dementia4. The strain of the disease not only affects patients but also has detrimental effects on their family members, who often quit their jobs and become full-time caregivers, but are drained by the physical and mental exertion, while simultaneously facing mounting financial difficulties. Individuals with posterior cortical atrophy (PCA) can no longer drive, and caregivers of FTD patients are often embarrassed by the sufferer’s public displays of inappropriate social behaviours12, 13. Thus, over 70% of caregivers experience significant stress and risk experiencing further psychological problems, a percentage much higher than that reported in caregivers for stroke patients8. Moreover, the support programs for old-age dementia are inappropriate or inaccessible for individuals with EOD that may leave them feeling lost3. Hence, each EOD is unique, and dementia cannot just be labelled under one blanket, for risk of undermining it’s the true extent of its impact.
Locally, the act of care for older adults is more of an ingrained nature and memory loss experienced by individuals is often accepted as a normal part of the ageing process12. However, due to the lack of awareness of specific symptoms of dementia such as aggressiveness or inappropriate sexual behaviours, patients may seek psychological support without consulting medical professionals12. Furthermore, these behavioural and neuropsychiatric symptoms of dementia (BPSD) are seen as burdensome despite being the most manageable facet of the disease12. With this in mind, it would not be difficult to imagine that EOD patients and their caregivers often face social stigma, particularly in Hong Kong, compared to those in other countries.
Driving Awareness to Ascertain a Resilient Future
A significant concern for individuals with EOD is the negative stereotype and fear attached to it, which presents a barrier to unhindered access to support services available to them. The shaming and blaming culture may further lead the sufferer to initially be in denial of the diagnosis and refuse assistance from healthcare professionals3. Thus, there is a need to provide articulate information that increases knowledge on the consequences of marginalisation among individuals with EOD, specifically by examining the barriers limiting the use of formal community services3. Undeniably, there is still a lot to do to support those with EOD, even just by simply integrating healthcare support, which is patient-centred14. In relation to this, a retrospective study by Damsgaard et al. (2023) evaluated the changes in healthcare utilisation before a young-onset Alzheimer's disease (YOAD) diagnosis. The study, including 1,082 YOAD patients and 3,246 controls, demonstrated that individuals with YOAD increased healthcare utilisation from 5 to 10 years prior to the diagnosis (Figure 1). Hence, the awareness of specific alterations in health-seeking behaviour may help healthcare professionals provide a timely diagnosis15.
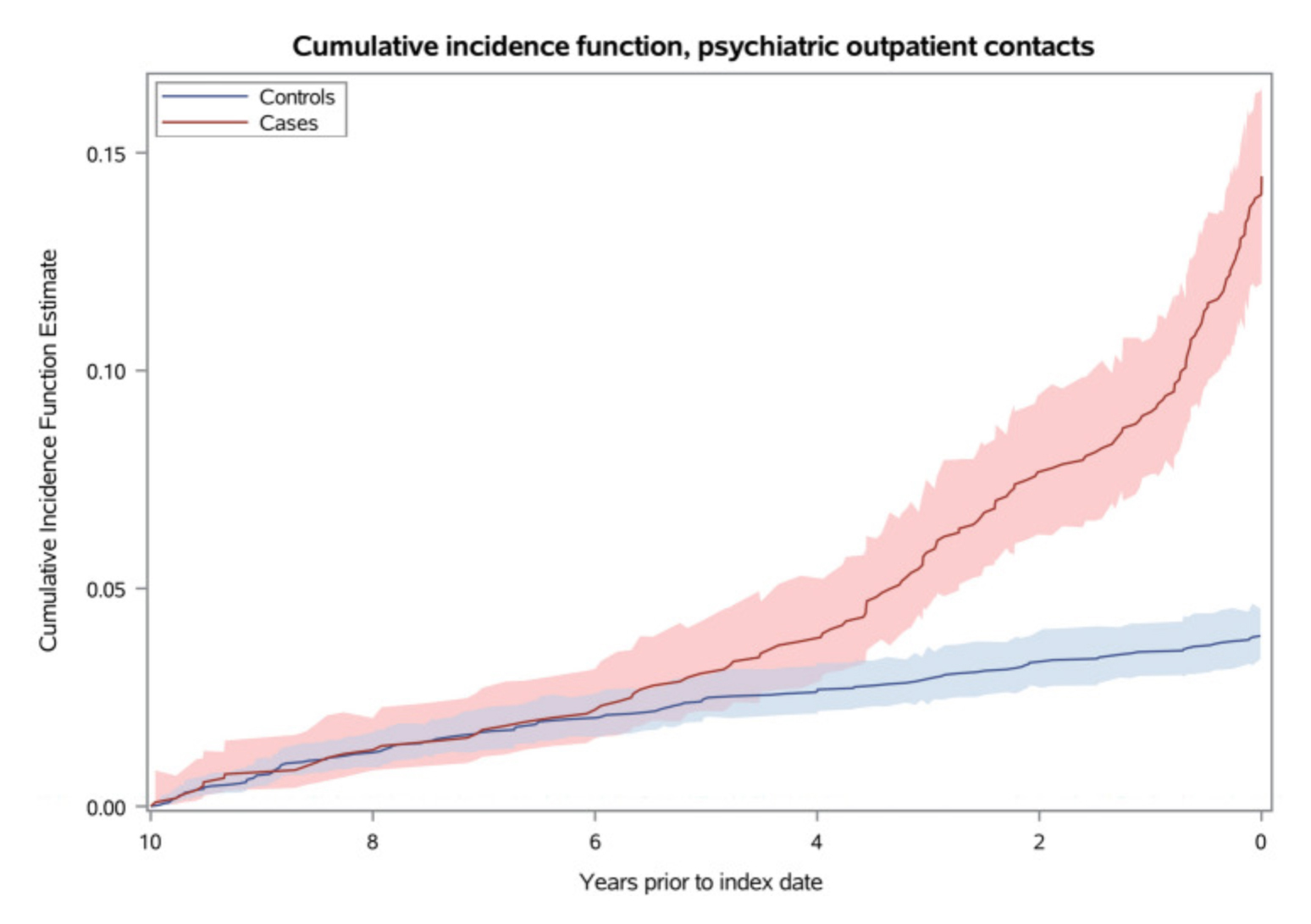
Figure 1. Cumulative incidence function, psychiatric outpatient contacts. The figure shows the cumulative incidence of psychiatric contacts for dementia cases and controls, respectively, over a 10-year period prior to the index date, which is the date of diagnosis for the dementia cases. The x-axis represents the time in years, and the y-axis represents the cumulative incidence of psychiatric outpatient contacts15.
The question that arises is whether there are any newer specific tests or biomarkers available to aid the diagnosis of dementia. The answer to this is yes: blood-based AD biomarkers, the neurofilament light chain (NFL) and amyloid beta-1-42 (Aβ1-42), are being tested and believed to have unique advantages that may transform and accelerate AD clinical trials16, 17. According to a longitudinal study, plasma NFL levels could be used as a non-invasive biomarker to track the neurodegeneration in patients with AD. However, NFL concentration is known to also increase in other neurodegenerative disorders, such as in FTD and LBD. Therefore, assessing both plasma NFL and Aβ1-42 is associated with higher diagnostic accuracy than assessing the individual biomarkers. Similar tests for AD have also been offered to the public by private healthcare providers recently18, and in tandem with the availability of lecanemab, an anti-amyloid monoclonal antibody with ‘disease-modifying’ capabilities19, this marks the advent of an improving biomedical landscape across all dementias. The next step is to understand proteomics and develop machine-learning algorithms that can also be applied to other dementias like FTD18. Even before signature biomarker panels for EOD are developed, these benefits will likely spill over to patients with EOD and offer more efficient ways to distinguish AD from non-AD EOD.
Although EOD may be creeping quietly and hidden, reaching a specific dementia diagnosis as well as the correct subsequent treatment and support is a priority that cannot be ignored. As the frontline, general practitioners must familiarise themselves with diagnosing EOD cases by conducting biomarker-based laboratory tests and neuroimaging tests rather than differentiating them through memory impairment and behavioural and psychological symptoms of dementia (BPSD) that are unspecific to EOD types20. For more complicated cases, referral to geriatricians, psychiatrists or neurologists, geneticists and neuroradiologists are needed to finally do away with ‘undetermined’ dementia categories. Awareness can ascertain a resilient future.
References
1. Fadil, H. et al. 2009. Int Rev Neurobiol 84:245–262. 2. Chiari, A. et al. 2021. Alzheimer’s & Dementia 17:81–88. 3. Nwadiugwu, M., 2021. Postgrad Med J 97:598–604. 4. Draper, B. et al. 2016. Int J Geriatr Psychiatry 31:1217–1224. 5. Lashley, T. et al. 2015. Neuropathology Appl Neurobio 41:858–881. 6. Leroy, M. et al 2021. Alzheimer’s Research & Therapy 13:19. 7. Yan, C.T.Y. and Tsoh, J.M.Y. 2016. Hong Kong Med J 42(2):35-43 8. CUHK. 2016. https://www.med.cuhk.edu.hk/press-releases/cuhk-sets-up-the-global-first-research-registry-on-early-onset-dementia-in-chinese-population 9. Outeiro, T.F. et al. 2019. Molecular Neurodegeneration 14:5. 10. Chu, L., 2017. Hong Kong Med J 23(3):218–9 11. Walker, L. et al. 2019. Journal of Neurochemistry 150:467–474. 12. Lam, T.P. 2019. Hong Kong Med J 25(6):473-482 13. Liu, K. et al., 2011. Hong Kong Med J 17:248-51 14. GovHK. 2022. https://www.info.gov.hk/gia/general/202206/01/P2022060100384.htm 15. Damsgaard, L. et al. 2023. J Neurol 270:6093–6102. 16. Schindler, S.E. et al. 2023. Alzheimers Dement 19:1175–1183. 17. Park, J.E. 2023. Biomedicines 10, 169. 18. Hall, 2023. Agetech World https://agetechworld.co.uk/news/blood-test-for-early-detection-of-alzheimers-launched-to-public/ 19. Edwards, M., Corkill, R. 2023. J Neurol 270:2342–2344. 20. Shea, Y.F. et al. 2015. Psychogeriatrics 15: 235–241.