Simon K Y Lee Professor in Gastroenterology
Chair of Medicine & Hepatology
Department of Medicine
The University of Hong Kong
A Change for Better Management of HBV Infection – When and How?
Viral hepatitis is a global public health problem: approximately 257 million people are living with chronic hepatitis B virus (HBV) and 71 million people are infected with chronic hepatitis C virus (HCV) globally1. In particular, the prevalence of HBV infection in Hong Kong is significant with 7.8% of the local population being infected with HBV2. Essentially, chronic hepatitis B (CHB)-related liver diseases constituted about 50% of all local cases of liver transplantation3. While tenofovir disoproxil fumarate (TDF) is one of the recommended medications for CHB patients, recent investigations suggested that tenofovir alafenamide fumarate (TAF) has a better safety profile as compared to TDF4. In a recent interview, Prof. Ching Lung Lai shared his expertise and clinical cases on managing CHB in order to demonstrate the health benefits of switching from TDF to TAF.
Pharmacological Advantages of TAF over TDF
Both TDF and TAF are recommended as the first-line therapy for CHB management5. However, TAF is more stable than TDF in plasma and delivers the active metabolite to hepatocytes more efficiently, allowing a lower plasma concentration than TDF6. A much lower amount of TAF is excreted through the kidneys compared to TDF. Thus, TAF would achieve similar antiviral activity with less systemic exposure and decreased renal and bone toxicity5.
As life-long treatment is often needed for CHB patients, the long-term safety is thus an important consideration in the therapeutic management. “Patients on TDF may develop renal complications including increase in creatinine and they may also develop bone complications including hypophosphatemia and osteopenia. However, these complications quite definitely would not occur in patients on TAF,” addressed Prof. Lai. He further advised that patients on TDF who have developed any signs of renal impairment or decrease in bone mineral density (BMD) should definitely switch to TAF. For treatment naïve patients, unless there is reason such as pregnancy, Prof. Lai recommended to start with TAF for managing CHB as well.
In response to the inquiry on efficacies of TAF and TDF in patients with multiple drug resistance, Prof. Lai noted that TDF is already very good for patients with multiple drug resistance. “TAF has at least the same efficacy, if not superior, as TDF in patients with multiple drug resistance. However, TAF causes much less complications, so I would prefer to use TAF,” he further commented.
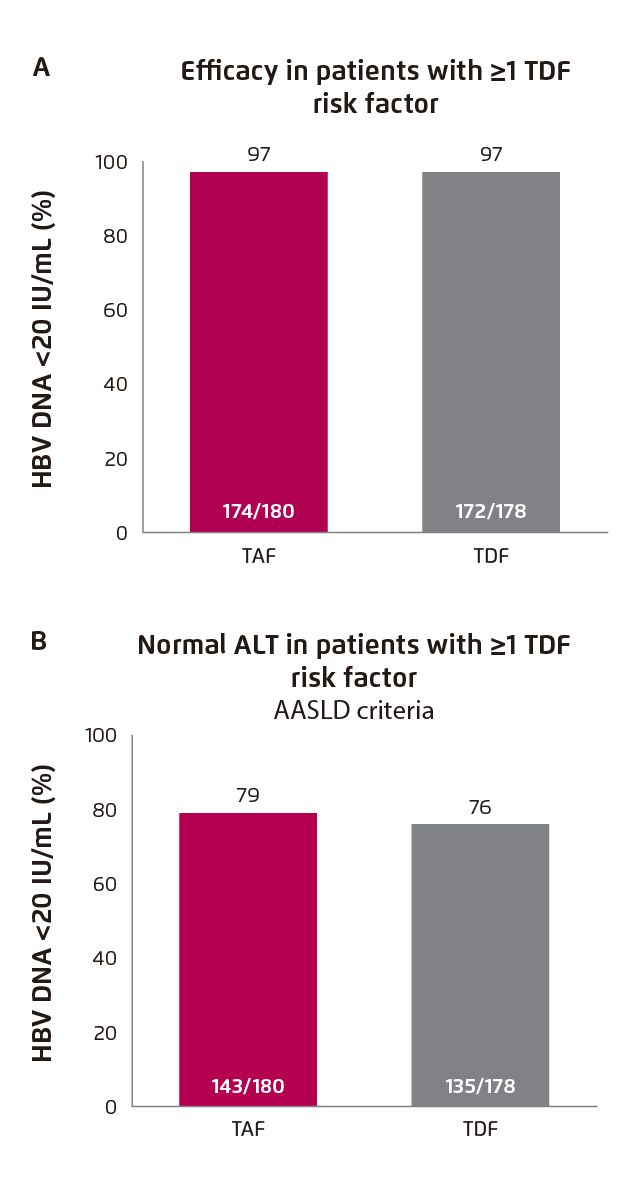
Figure 1. Treatment outcomes of switching from TDF to TAF. (A) Viral suppression and (B) ALT normalisation4
Evidence on Benefits of Switching from TDF to TAF
The treatment benefits of switching from TDF to TAF were demonstrated in the Study 4018, a recent Phase III randomised control trial involving 488 CHB patients. The patients were virologically suppressed on TDF and were randomly assigned to switch to TAF (n=243) or continue TDF (n=245) for 48 weeks. Among the subset of patients with TDF risk factor, viral suppression was maintained after switching from TDF to TAF (97% [TDF to TAF] vs. 97% [TDF]), whereas numerically higher rate of ALT normalisation was observed in TAF group (79% [TDF to TAF] vs. 76% [TDF]) at week 48 (Figure 1A and 1B)4.
The impact of switching medication on renal function and bone were evaluated in Study 4018. In patients with TDF risk factor, significant improvement in renal function, in terms of change in estimated glomerular filtration rate (eGFR) from baseline, was achieved in patients who switched from TDF to TAF (1.86 mL/min [TDF to TAF] vs. -2.70 [TDF], p<0.001, Figure 2). Besides, switching medication also yielded significant improvement in hip and spine BMD (hip BMD: 0.67 [TDF to TAF] vs. -0.53 [TDF], p<0.001, Figure 3A; spine BMD: 1.81 [TDF to TAF] vs. -0.33 [TDF], p<0.001, Figure 3B)4.
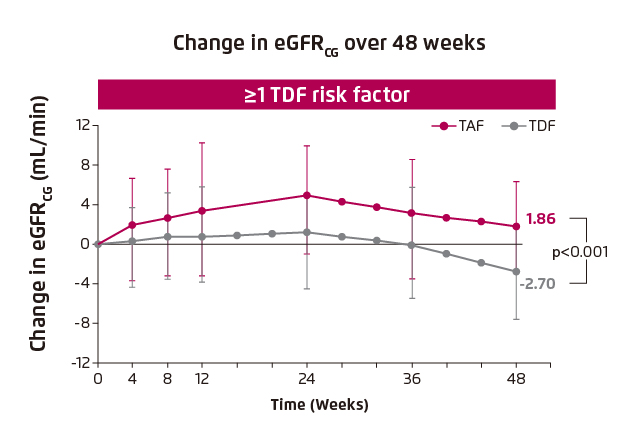
Figure 2. Improvement in renal function in patients with TDF risk factors4
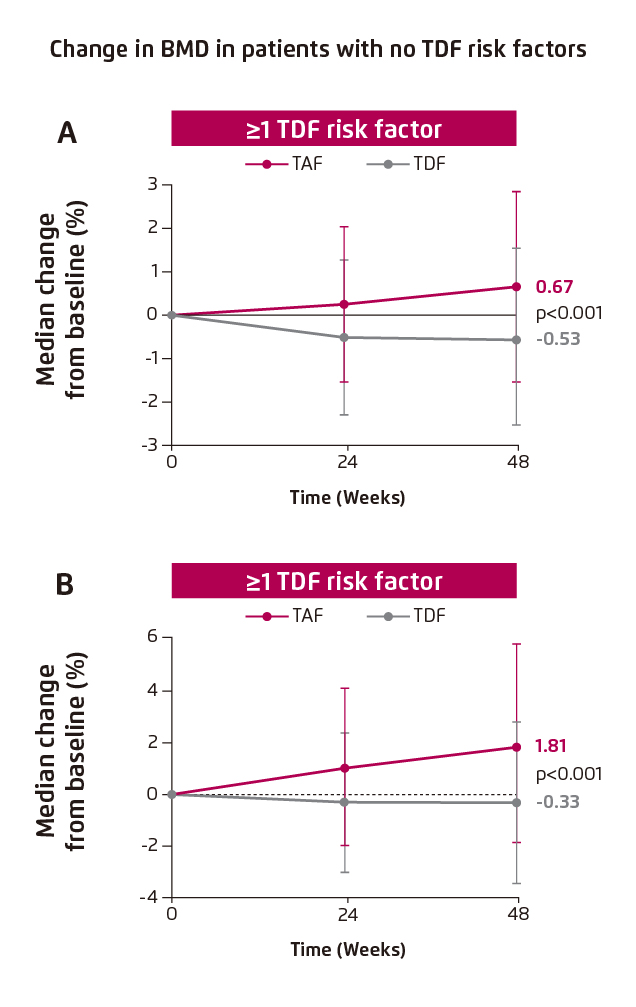
Figure 3. Improvement in BMD in patients with TDF risk factors, (A) hip BMD and (B) spine BMD.4
BMD: bone mineral density.
In addition, one of the most important findings in Study 4018 was the improvement in bone quality upon switching from TDF to TAF in patient with no TDF risk factor. Significant improvement in hip and spine BMD was found (hip BMD: 0.64 [TDF to TAF] vs. -0.46 [TDF], p<0.001, Figure 4A; spine BMD: 1.52 [TDF to TAF] vs. 0.48 [TDF], p=0.04, Figure 4B)4.
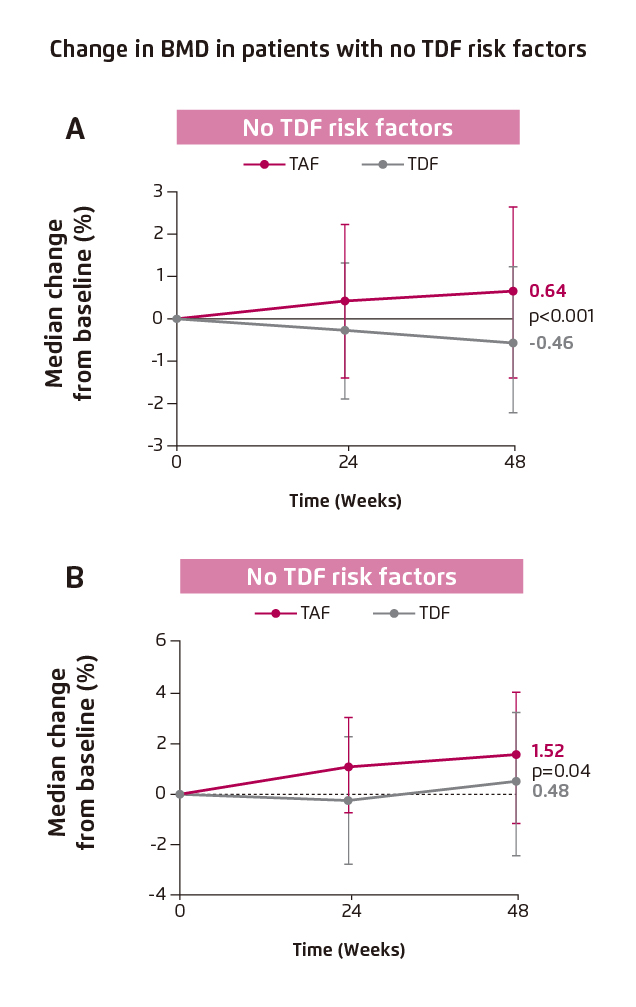
Figure 4. Improvement in BMD in patients with no TDF risk factors, (A) hip BMD and (B) spine BMD4
“HBV carriers are prone to BMD decline as compared to the general population,” noted Prof. Lai. However, bone mineral density testing is currently not a routine test for HBV carriers in the Hospital Authority practice. Hence, Prof. Lai agreed that there may be under-diagnosis of decline in BMD among CHB patients. Nonetheless, he emphasised that patients on any drug which may cause osteopenia should have their BMD tested every year. Importantly, in CHB patients without TDF risk factors, such as obesity and age>60, Prof. Lai advocated switching from TDF to TAF for this group of patients, even without the sign of decline in renal function and BMD. “They may benefit before renal complications and BMD decline actually develop. Also, there is another benefit for switching from TDF to TAF. ALT normalisation would be improved with TAF as compared to TDF, and better ALT normalisation is associated with decreased risk of hepatocellular carcinoma (HCC),” highlighted Prof. Lai.
Switching to TAF in Practice
Prof. Lai illustrated the efficacies of switching from TDF to TAF in CHB management with the case of a 42-year-old male patient. The patient was treated with TDF for at least 3 years and had developed renal complication as indicated by an increase in creatinine. Fortunately, the levels of phosphate and calcium were normal. Prof. Lai switched the medication from TDF to TAF for the patient due to the detected renal complication. Within 3 months since the switching to TAF, the creatinine level improved from slightly elevated to normal level, while the eGFR, phosphate and calcium levels remained normal. Essentially, the viral suppression was well maintained and the viral load was below detection limit. Also, the patient tolerated the TAF treatment well, with no complaint of any side effects.
Final Remarks
In the practical switching from TDF to TAF, Prof. Lai commented that TDF is not as safe as TAF and thus no specific precaution is needed in the switching process. Except for pregnant women, TDF is still the only medication licensed for viral suppression in pregnant women or patients planning to be pregnant. In response to the inquiry on practical procedures for switching from TDF to TAF, Prof. Lai highlighted the simplicity in the switching of medication in that no dosage adjustment is needed. Moreover, Prof. Lai added that TAF is safe for patients with end stage renal diseases as well.
Summarising the previous trial data, switching from TDF to TAF will not only maintain viral suppression, but also improve renal function and BMD. In particular, even in patients with no TDF risk, better treatment outcomes are expected upon switching to TAF as compared to TDF. As recommended by Prof. Lai, prescribing TAF is preferred for both TDF-treated and treatment-naïve CHB patients.
References
1.World Health Organization. Global Hepatitis Report 2017. 2. Wong et al. Surveillance of Viral Hepatitis in Hong Kong - 2016 Update Report. 3. Lo et al. Hong Kong Med J. 2002;8:240-244. 4. Buti et al. AASLD 2019.:476. 5. Terrault et al. Hepatology. 2018;67(4):1560-1599. 6. Agarwal et al. J Hepatol. 2015;62(3):533-540.