A Great Leap forward in Therapeutic Development
Discoveries of molecular mechanisms of different diseases provide unprecedented opportunities to develop novel medicines. However, the research and development of a brand-new drug is time-consuming and costly1. Regarding the possible bottlenecks de novo drug development, drug repurposing is an innovative and economical approach to reduce the time frame and investment costs1. Applications of drug repurposing may also open up a golden opportunity for the treatments for rare diseases and drive a paradigm shift in drug development.
A New Horizon in Drug Development
“The most fruitful basis for the discovery of a new drug is to start with an old drug,” highlighted Sir James Whyte Black, the winner of the 1988 Nobel Prize in Physiology or Medicine2. With their well-documented pharmacological properties and clinically proven safety profiles, marketed drugs emerge as the treasure troves in drug development. Drug repurposing is an unconventional strategy which redirects the therapeutic indications of these approved drugs that are outside the scope of their original indications1. For the past decades, drug repurposing has become increasingly well-known. Familiar examples include thalidomide for multiple myeloma and sildenafil for erectile dysfunction, which are originally designed for treating morning sickness during pregnancy and angina respectively2.
Key Benefits of Drug Repurposing
De novo drug development is a labourious journey that requires a vast amount of time and effort. Before getting the approval from regulatory authorities and being launched on the market, the new chemical entity has to be evaluated in several stages of the whole conventional drug development, ranging from laboratory testing to different phases of clinical trials3. For instance, the de novo drug development for cancer treatment is estimated to take 10-17 years3. In contrast, drug repurposing for cancer therapy takes only 3-9 years as it bypasses the first few steps of the drug development with the prior well-established drug profiles of the drug candidates3 (Figure 1).
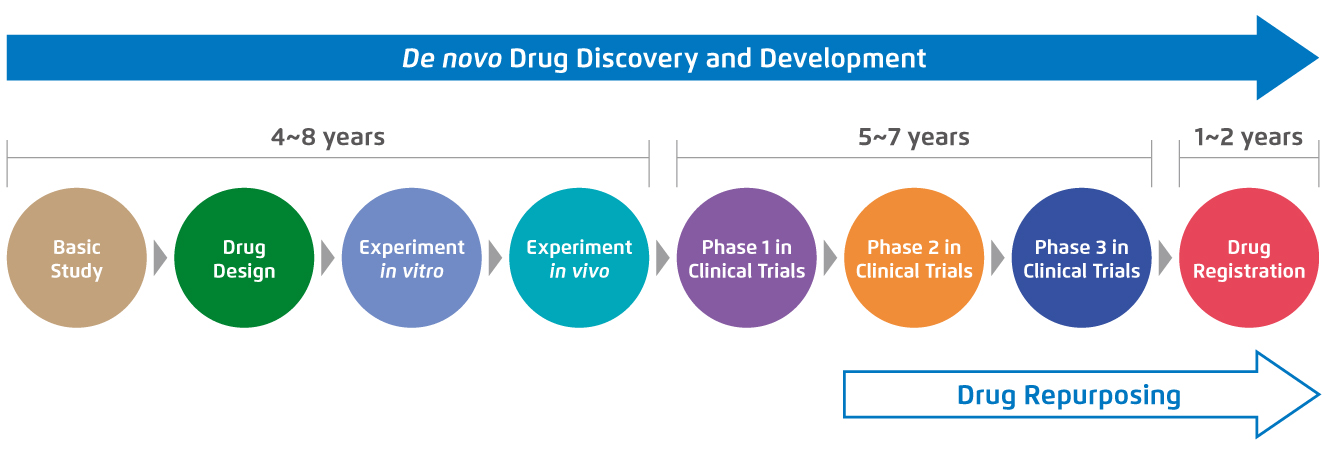
Figure 1.
Standard procedures and the required time of de novo drug development compared with drug repurposing for cancer treatment3.
Computational approach of drug repurposing may also offer a relatively quick method to integrate diverse data and identify precise therapeutic targets1. Among different approaches of computational drug repurposing, network-based approaches have been extensively utilised which focus on the networks between drugs, therapeutic targets and diseases and it helps visualising the large-scale and complex relationships among molecules that may accelerate the progress of drug repurposing4. The advancements of large-scale genome sequencing potentially provide opportunities to enrich the understanding of the genetic variations and identify particular biological targets, which also enables accelerated target identification and minimises the translational gap between genomic medicine studies and clinical outcomes1,5.
Owing to the emergence of side effects or adverse events, a vast amount of de novo drugs fails to get through clinical trials and gain regulatory approval6. As the marketed drugs have already been found to be sufficiently safe in preclinical models and humans, the failure rate may be partly diminished from the safety perspective when repurposing those drugs1. In addition, compared with the de novo drug development, drug repurposing can result in substantial savings with lower expenses (estimated US$300 million on average versus nearly US$2-3 billion needed for a new chemical entity to be launched on the market1). Given the high attrition rate and enormous investment cost of de novo drug development, drug repurposing is considered as an appealing alternative for both common and rare diseases with potentially lower expenditure involved, especially repurposing off-patent drugs1.
Drug Repurposing in Rare Diseases: Opportunities and Challenges
Above 7,000 rare diseases exist worldwide nowadays, yet more than 95% of them lack FDA-approved treatment1. Regarding the disease heterogeneity, limited number and dispersed distribution over a wide geographic area of the patients, the reluctance of pharmaceutical companies to start de novo drug development probably can be attributed to inadequate understanding of pathophysiology or biological pathways correlated with the disease development1,7. For this reason, rare diseases are also known as orphan diseases8. Commercially, as the cost of research and development of a novel drug can only be amortised over a comparatively small number of patients, the financial disincentive can be one of the significant hindrances as well1.
Drug repurposing may be a promising strategy to address the current translational gap in rare disease drug development with examples that the marketed drugs have obtained additional approvals for rare diseases9. As a result of drug repurposing, sildenafil, a selective inhibitor of cGMP-specific phosphodiesterase-5 that had been initially designed as an anti-angina drug, was later approved for erectile dysfunction treatment in 19989. On the basis of its vascular effect, sildenafil was also subsequently repurposed for pulmonary arterial hypertension, a rare disease with low diagnosed cases each year in United States, and leading to the approval for this indication in 20059-11.
Despite the advantages of drug repurposing, multifaceted challenges from different perspectives cannot be neglected. For instance, the clinical safety of repurposed drugs may drastically change in the presence of different sets of conditions characterising the new indication or treatment regimen9. Additionally, underlying worries may still exist owing to the insufficient time for pharmaceutical companies to recoup the investment cost even with the prolonged period of data exclusivity extended by regulatory authorities9,12. Accordingly, collaborations between pharmaceutical firms, biotechnology companies and other stakeholders are vital to drive the field of drug repurposing, particularly exploring opportunities of marketed drugs repurposed for rare diseases.
A Next Step in Drug Development
Drug repurposing has been progressively growing in recent decades, which revolutionises the drug development process by shortening the time frame and lowering the investment cost1,3. Rare diseases are estimated to collectively affect around 350 million patients worldwide9. Recently, a number of novel predicted associations related to a rare disease, adrenocortical carcinoma, appear to be valid and the predicted relations are expected to be beneficial for expediting the necessary clinical processes13. Developing clinical treatments for rare and neglected diseases may not be profitable in terms of economic returns, but it implies that other set of values including corporate social responsibility of pharmaceutical companies are fostered. The joint efforts of different stakeholders may help realise the full potential of drug repurposing and alleviate the suffering of patients affected by rare diseases globally.
References:
1. Pushpakom S, et al. Nat Rev Drug Discov 2019;18:41-58. 2. Zhou Y, et al. Lancet Digit Health 2020;2:e667-e676. 3. Zhang Z, et al. Signal Transduct Target Ther 2020;5:113. 4. Yella JK, et al. Pharmaceuticals (Basel). 2018;11:57. 5. Cheng F, et al. Nat Commun 2019;10:3476. 6. Saberian N, et al. Bioinformatics 2019;35:3672-3678. 7. Whicher D, et al. Orphanet J Rare Dis 2018;13:14. 8. National Center for Advancing Translational Sciences. FAQs About Rare Diseases. Available at https://rarediseases.info.nih.gov/diseases/pages/31/faqs-about-rare-diseases. (Accessed on 26 February 2021). 9. Tambuyzer E, et al. Nat Rev Drug Discov 2020;19:93-111. 10. American Lung Association. Learn About Pulmonary Arterial Hypertension. Available at https://www.lung.org/lung-health-diseases/lung-disease-lookup/pulmonary-arterial-hypertension/learn-about-pulmonary-arterial-hypertension. (Accessed on 26 February 2021). 11. National Organization for Rare Disorders. Pulmonary Arterial Hypertension. Available at https://rarediseases.org/rare-diseases/pulmonary-arterial-hypertension/. (Accessed on 1 March 2021). 12. Talevi A, Bellera CL. Expert Opin Drug Discov 2020;15:397-401. 13. Lotfi Shahreza M, et al. Sci Rep 2020;10:8846.