Heart failure is a complex and progressive clinical syndrome that affecting 1-2% of general adult population worldwide. Despite advances in both the pharmacological and the device-based management, heart failure remains a leading cause of hospitalisation and mortality worldwide1. This highlights the urgent need for effective and safe therapies for the disease. Originally investigated as glucose-lowering agents, sodium-glucose cotransporter 2 inhibitors (SGLT2i) have been demonstrated to prevent the development of heart failure regardless of diabetes status. The clinical benefits of SGLT2i were shown to be independent of their glucose-lowering effect2. In particular, the results of the EMPEROR-Preserved trial demonstrated that empagliflozin, a SGLT2i, can significantly reduce hospitalisation due to heart failure3. The objective of this article is to review the recent breakthrough in the pharmacological management of heart failure with SGLT2i.
Beyond Glycaemic Control
SGLT2i is a class for therapies which lowers glucose burden by inhibiting the major transporter involved in glucose reabsorption in kidney and hence increasing urinary glucose excretion4. While the risk of hypoglycaemic events is a major concern for many glucose-lowering medications, the hypoglycaemic risk for SGLT2i is lower due to its insulin-independent mechanism of action5. On the other hand, the increased excretion of glucose and associated calories by SGLT2i leads to reduction in body weight6.
In addition to the efficacy in glycaemic control, the cardiovascular (CV) benefits of SGLT2i in patients with type 2 diabetes mellitus (T2D) have been well-established. For instance, in the EMPA-REG OUTCOME trial, which was a randomised controlled trial (RCT) involved totally 7,020 T2D patients at high-risk for CV events, the effects of empagliflozin in addition to standard care on CV morbidity and mortality were evaluated. After a median observation time of 3.1 years, empagliflozin yielded superior efficacy in lowering the rate of death from CV causes (10.5% vs 12.1%, p =0.04, Figure 1) and of death from any cause (5.7% vs 8.3%, p <0.001, Figure 2). Although genital infection were common upon empagliflozin treatment but there was no increase in other adverse events. Thus, the results provided strong evidence on the reduction of CV risk by empagliflozin7.
Cardioprotective Effects Regardless of Diabetes Status
The remarkable efficacy of SGLT2i demonstrated in previous RCTs with T2D patients7 led to further investigations on SGLT2i in patients with heart failure without T2D. The EMPEROR-Reduced Trial was one of the landmark trials in this regard. In the trial, 3,730 patients with heart failure with reduced ejection fraction ([HFrEF]: left ventricular [LV] ejection fraction [LVEF] ≤40%, New York Heart Association [NYHA] class II–IV), were randomly assigned to receive empagliflozin or placebo in addition to recommended therapy. After a median of 16 months follow-up, a composite of CV death or hospitalisation for worsening heart failure, i.e. the primary outcome, occurred in 19.4% of patients treated with empagliflozin, which was significantly less than that in the placebo group (24.7%,p <0.001, Figure 3). Of note, the effect of empagliflozin on the primary outcome was consistent in patients regardless of diabetes status. Moreover, the total number of hospitalisations for heart failure was lower in the empagliflozin group than in the placebo group (hazard ratio [HR]: 0.70, p <0.001, Figure 4)8. The results thus indicated that empagliflozin with recommended therapy for heart failure would lower the risk of CV death or hospitalisation for patient with HFrEF, irrespective of diabetes status.
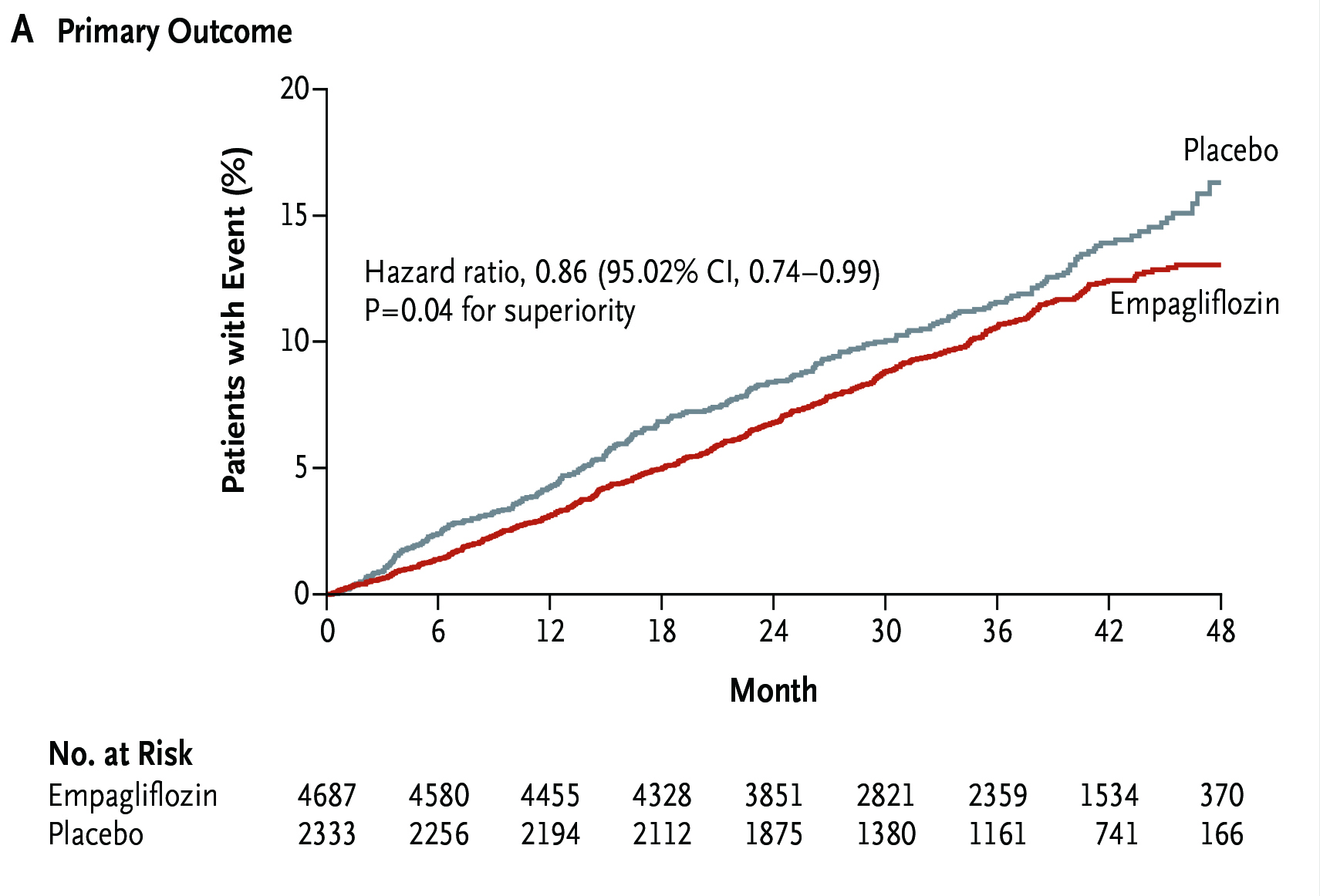
Figure 1. Reduced rate of primary outcome achieved by empagliflozin7
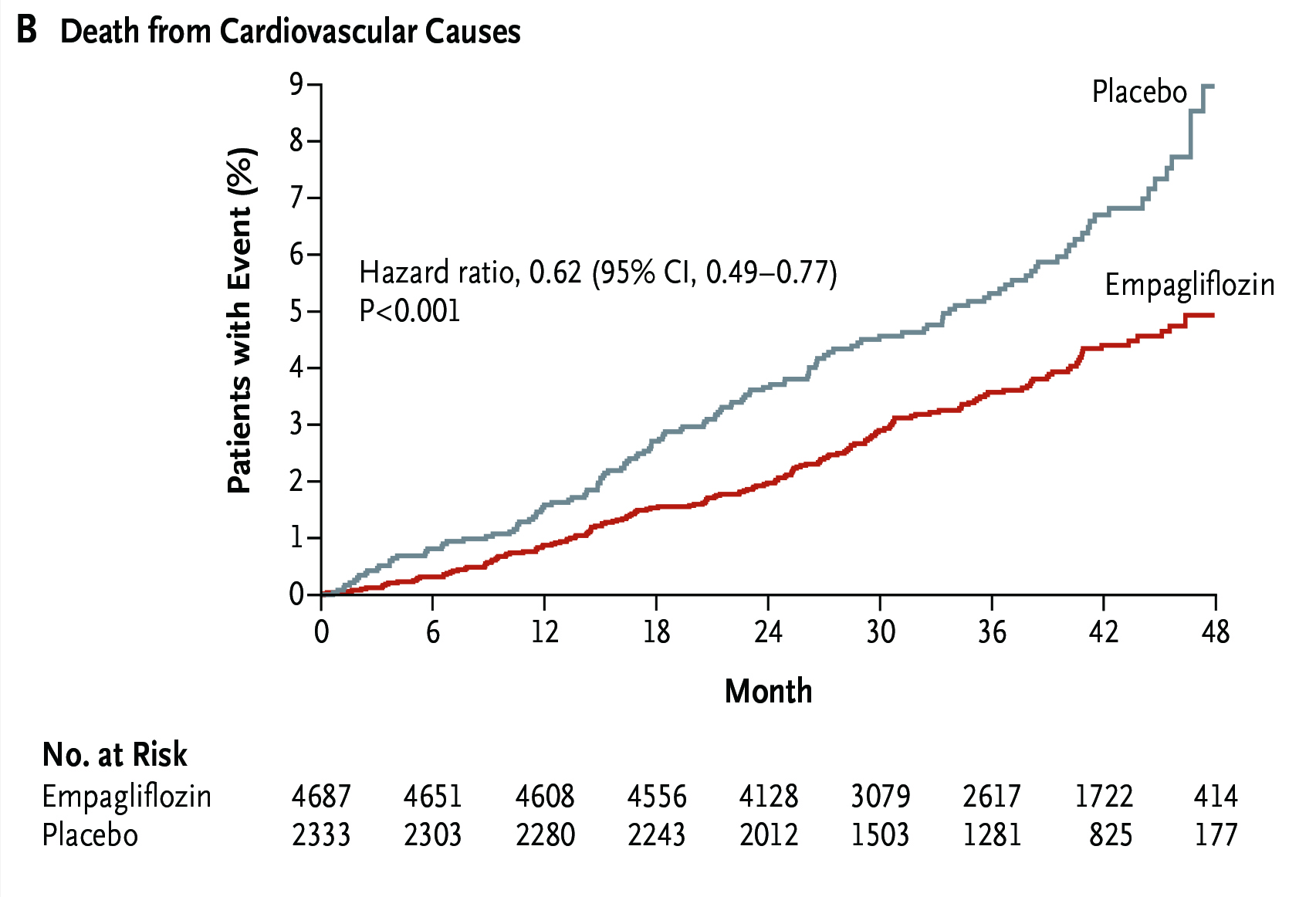
Figure 2. Reduced rate of death from all causes achieved by empagliflozin7
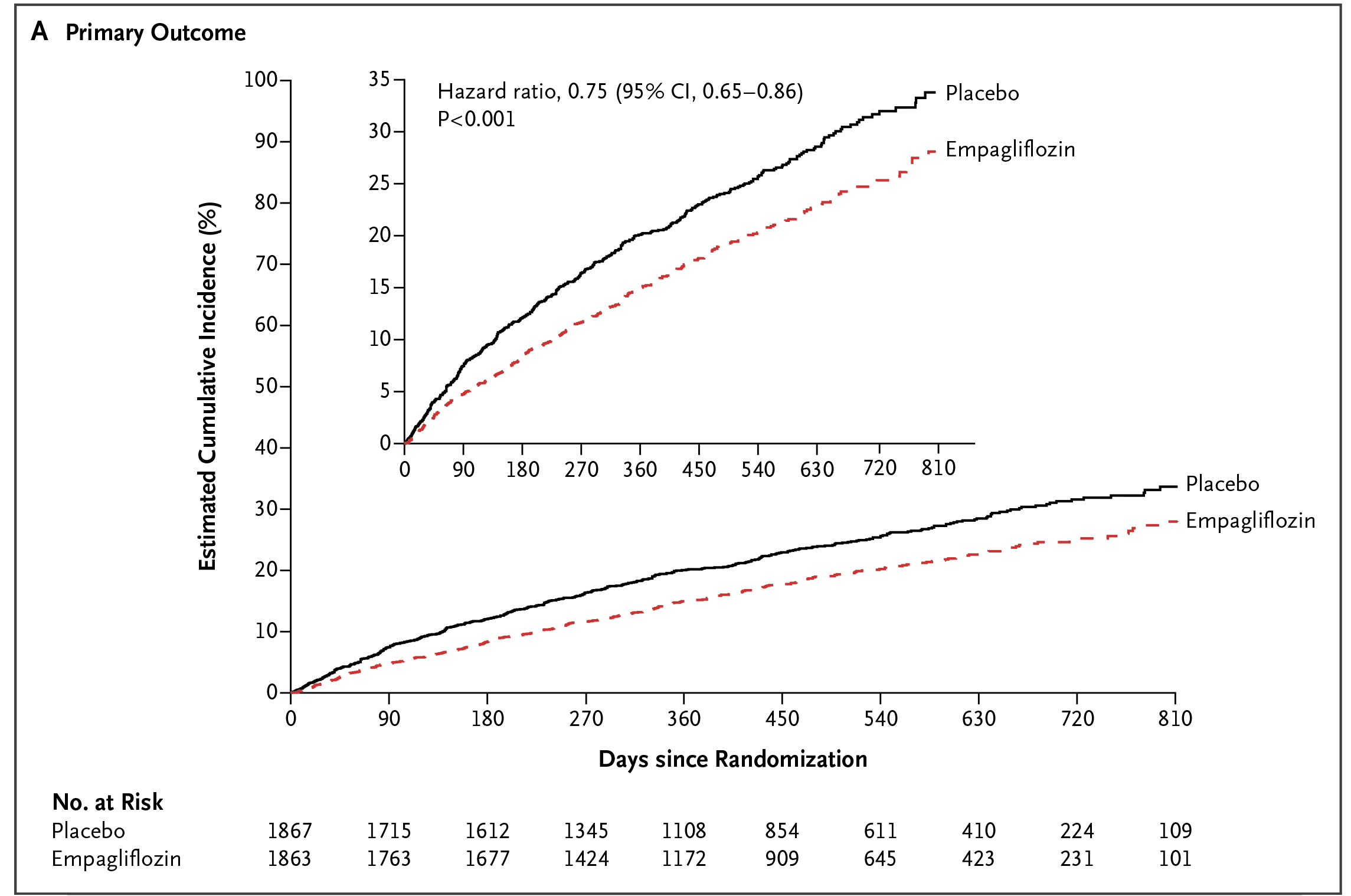
Figure 3. Lower incidence of primary outcome achieved by empagliflozin8
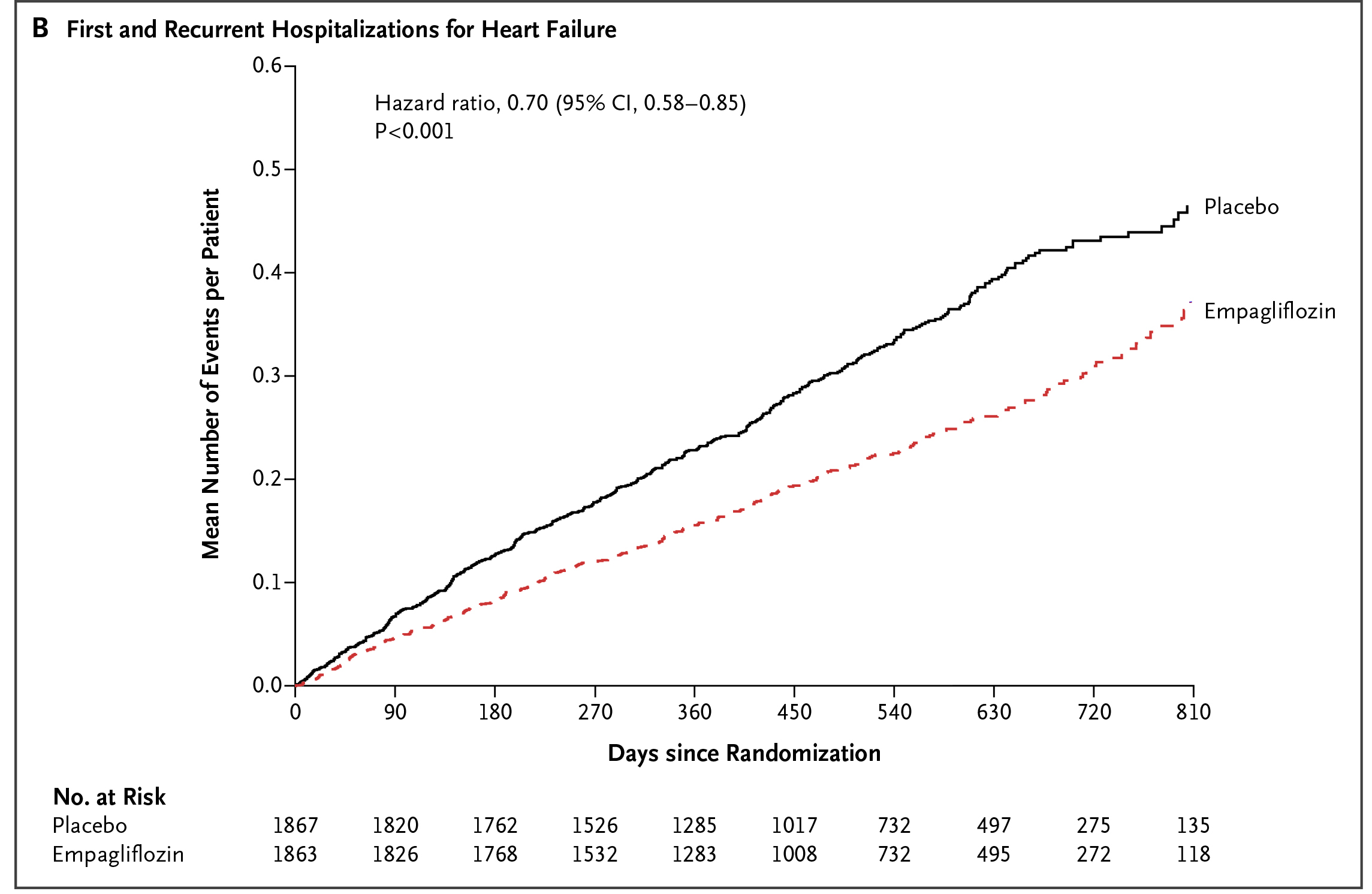
Figure 4. Lower number of hospitalisation for heart failure achieved by empagliflozin8
The CV benefits of SGLT2i was further supported in a recent meta-analysis by Cardoso et al (2021) including 15 randomised trials accounting for 20,241 patients with heart failure, among which 52.3% received SGLT2i. The results indicated that all-cause mortality (HR: 0.86, p =0.0007) and CV mortality (HR: 0.86, p =0.006) were significantly lower in patients treated with SGLT2i compared with placebo. Moreover, the composite of CV mortality, heart failure hospitalisations, or urgent visits for heart failure was significantly reduced with SGLT2i in all the subgroup analyses9. These findings thus confirmed the efficacy of SGLT2i in reducing mortality and hospitalisation in patients with heart failure.
Recent Breakthrough in the Management of Heart Failure with Preserved Ejection Fraction
Apart from HFrEF, heart failure with preserved ejection fraction (HFpEF), which accounts for about 50% of all patients with heart failure, has been a diagnostic and therapeutic challenge. Conventional therapies for HFpEF focus mainly on management of symptoms and comorbidities10. Whereas, the recent findings in the EMPEROR-Preserved trial has enlightened the pharmacological management of HFpEF.
In the trial, 5,988 patients with symptomatic HFpEF were randomly assigned to receive empagliflozin (n =2,997) or placebo (n =2,991) in addition to usual therapy. After a median of 26.2 months follow-up, the primary composite outcome, i.e. CV death or heart failure hospitalisation, was significantly lower in the empagliflozin group compared to the placebo group (13.8% vs 17.1%, p <0:001, Figure 5). The results further indicated that empagliflozin treatment yielded significantly lower total number of hospitalisations for heart failure than the placebo group (HR: 0.73, p <0.001). Besides, the rate of decline in the estimated glomerular filtration rate (eGFR) was significantly slower in the empagliflozin group as compared with the placebo group (-1.25 vs -2.62 ml/min/1.73m2 per year, p <0.001). Nonetheless, the difference in CV death numbers were not significant between the 2 groups. In view of safety profile, the percentage of patients who discontinued treatment for reasons other than death was similar in the 2 treatment groups3.
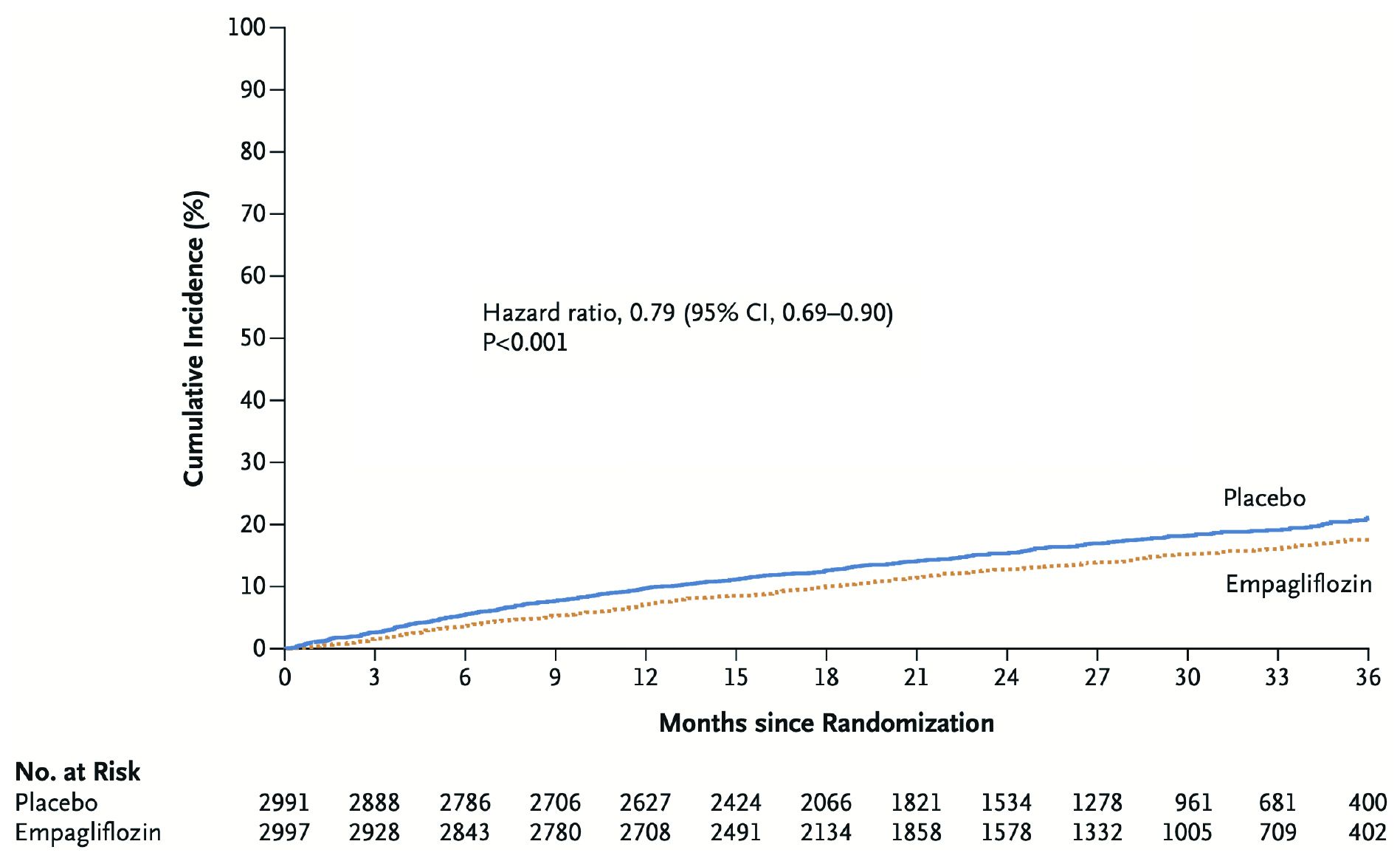
Figure 5. Estimated cumulative incidence of the primary outcome in the EMPEROR-Preserved trial3
Integrating SGLT2i in the Management of Heart Failure
As demonstrated in the growing body of clinical evidence, SGLT2is are beneficial when added to current standard of care for the treatment of heart failure regardless of diabetes. The therapies are generally well-tolerated and considered to have a favourable risk–benefit profile. Hence, the SGLT2i has been advocated as one of the four classes of therapies for all HFrEF patients to reduce mortality in the ESC Guidelines 202111. As the first RCT investigating the efficacy and safety of SGLT2i in HFpEF, the EMPEROR-Preserved trial is a milestone in the pharmacological development for the disease. The trial results have proven that empagliflozin is a safe and effective therapy for reducing heart failure hospitalisation in patients with HFpEF. To optimise the outcomes of heart failure management, former opinion suggested that a clinical practice algorithm considering a patient’s unique profile of heart failure-related risk factors and concomitant medications to guide the selection of SGLT2i therapy would be required in integrating SGLT2i into the clinical management.
References
1. Lytvyn et al. Circulation 2017; 136: 1643-V58. 2. Docherty et al. Heart 2022; 108: 312-V20. 3. Anker et al. N Engl J Med 2021; 385: 1451-V61 4. List et al. Kidney Int 2011; 79: S20-V7. 5. Gerich. Diabet Med 2010; 27: 136-V42. 6. Bolinder et al. J Clin Endocrinol Metab 2012; 97: 1020-V31. 7. Zinman et al. N Engl J Med 2015; 373: 2117-V28. 8. Packer et al. N Engl J Med 2020; 383: 1413-V24. 9. Cardoso et al. eClinicalMedicine 2021; 36: 100933. 10. Toth et al. Postgrad Med 2021; 133: 125-V39. 11. McDonagh et al. Eur Heart J 2021; 42: 3599-V726.