Global human immunodeficiency virus (HIV) control has accelerated, yet uneven access and financing shocks threaten recent gains. In 2024, an estimated 40.8 million people were living with HIV; 77% received antiretroviral therapy (ART) and 73% had suppressed viral loads—contributing to dramatic reductions in new infections and deaths since 2010.1 At the same time, innovation has shifted from daily management toward lighter, longer‑acting regimens and credible steps towards cure. Twice‑yearly lenacapavir for pre‑exposure prophylaxis (PrEP) gained regulatory approvals in 2025 after exceptional trial efficacy, while Cabenuva (cabotegravir/rilpivirine) expanded monthly or every‑other‑month treatment options with real‑world effectiveness. Immune‑based strategies—including broadly neutralizing antibodies (bNAbs) paired with lenacapavir, CRISPR gene editing, and mRNA latency reversal—are advancing, moving cure research from concept to cautious human studies. Yet UNAIDS warns that 2025 funding shocks are disrupting prevention programs, underscoring an urgent need for sustainable financing.2 This article synthesizes the latest global statistics, long‑acting tools, cure‑oriented science, patient‑centered formulations, and access realities—ending with a discussion of health policy and financing.
At the end of 2024, the world counted 40.8 million people living with HIV (PLHIV). ART coverage reached 31.6 million (77%), and 73% of PLHIV achieved viral suppression. New HIV infections fell by 40% since 2010; and acquired immunodeficiency syndrome (AIDS)‑related deaths dropped by 54% (from 1.4 million to 630,000). Compared to the peak in 2004, the global HIV epidemic claimed 70% fewer lives in 2024 —landmarks achieved through testing, treatment scale‑up, and prevention (Figure 1).1
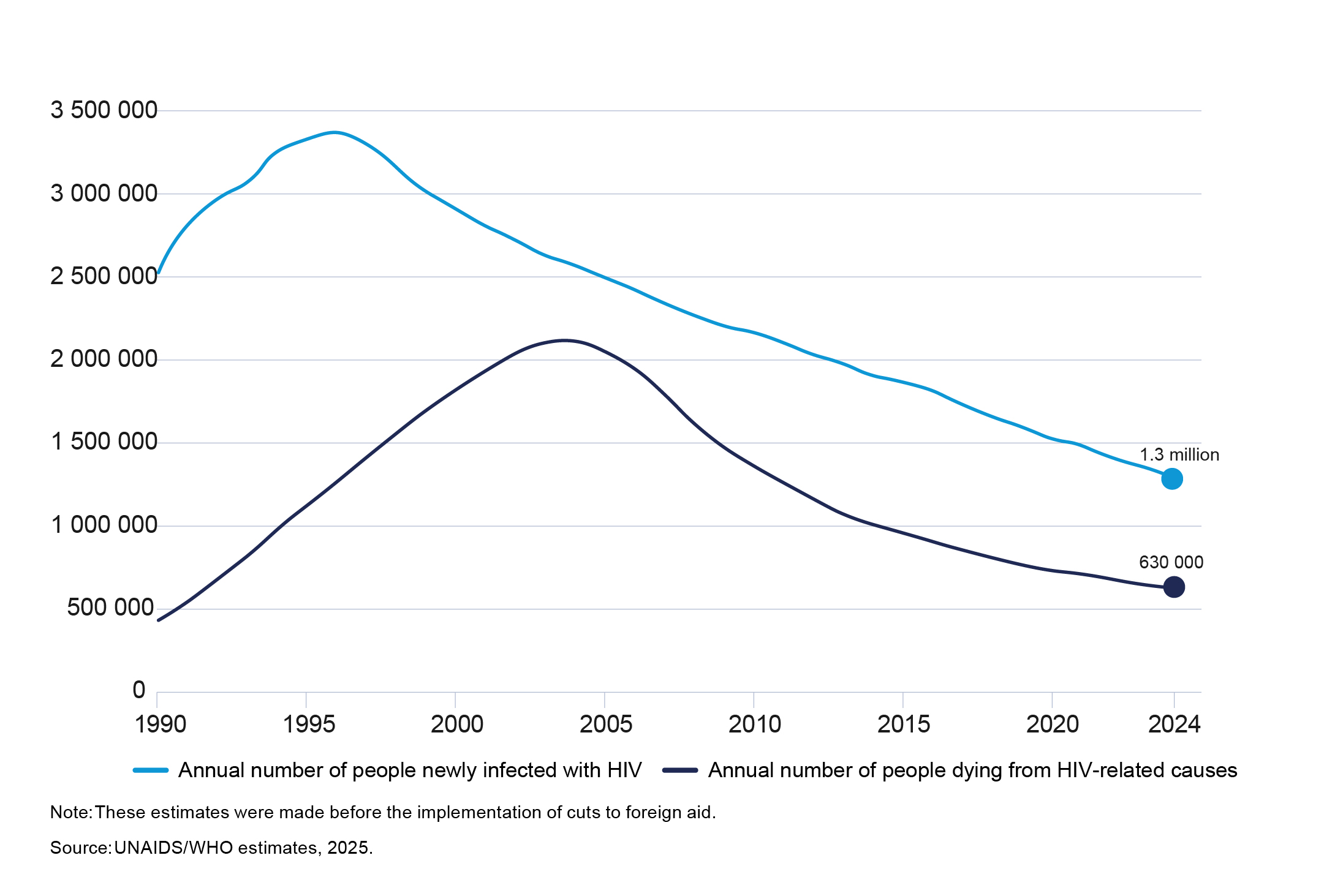
Figure 1: Global trends in people acquiring HIV and people dying from HIV-related causes (1990–2024)1
These gains coexist with significant regional contrasts. For example, China saw a 15‑fold rise in newly reported cases between 2005 and 2019. While HIV/AIDS-related deaths in China rose more modestly—by 25%, from 40,711 in 2005 to 51,250 in 2019—this slower growth may be largely attributed to improved access to antiretroviral drugs. Nevertheless, China continues to face significant challenges in curbing transmission and managing the evolving epidemic.3
Although substantial progress has been achieved, enduring challenges underscore the need for transformative treatment strategies and integrated health policy frameworks to accelerate the path toward an HIV cure.
In June 2025, the U.S. FDA approved lenacapavir as the first twice‑yearly injectable PrEP, backed by PURPOSE 1 and PURPOSE 2 phase‑3 trials that demonstrated near‑complete protection when administered as directed.4,5 WHO welcomed the approval and issued global guidelines in July 2025 recommending lenacapavir as an additional PrEP option, highlighting its potential to overcome adherence barriers.6
Roll‑outs followed in several countries, with WHO prequalification and national fast‑track authorizations enabling access, particularly across parts of Africa. Early implementation has underscored both transformative potential and urgent affordability/financing questions, given funding disruptions and price debates.7
What makes lenacapavir different? Two subcutaneous injections every six months, preceded by a brief oral loading dose, can initiate protection rapidly; high adherence is achievable because the regimen minimizes clinic touchpoints—an advantage for people who struggle with daily oral PrEP.8,9
Long‑acting cabotegravir for PrEP laid critical groundwork for injectable prevention from 2021 onward, and manufacturers have tripled supply commitments for low‑ and middle‑income countries in 2025–2026, under not‑for‑profit pricing in prioritized geographies. These actions complement lenacapavir’s entry, broadening choices across daily, bi‑monthly, and twice‑yearly models of prevention.10
For treatment, Cabenuva enables monthly or every‑other‑month injections as a complete regimen for virologically suppressed patients aged ≥12 years—with dosing schedules validated in labeling and guidance. Clinical experience shows comparable safety and effectiveness across dosing intervals and practical pathways for missed injections—key for real‑world continuity.11
To reach twice‑yearly treatment, developers are pairing lenacapavir with bNAbs. Phase‑2 studies presented at CROI 2025 and EACS 2025 indicate that lenacapavir + teropavimab + zinlirvimab can maintain suppression in carefully selected, bNAb‑sensitive patients—hinting at future six‑monthly regimens if resistance screening, dosing optimization, and access logistics are solved.12
bNAbs target conserved regions of HIV’s envelope, neutralizing diverse strains. Recent clinical studies report post‑intervention control for subsets of participants after pausing ART, implicating stem‑like CD8+ T‑cell qualities that synergize with antibody therapy. These results—though not generalized yet—validate a path toward functional remission supported by immune mechanisms.13
Early human trials (e.g., EBT‑101) using CRISPR‑Cas systems to target integrated proviral DNA show promising safety and on‑target activity; while viral rebound occurred after ART interruption in most participants, delayed rebound and reservoir decreases in at least one case encourage next‑generation vectors and improved delivery. Parallel laboratory advances continue to demonstrate excision of HIV DNA from infected cells and protection against reinfection, setting the stage for safer, scalable delivery strategies.14
A major barrier to cure is the latent reservoir in resting CD4+ T cells. In 2025, researchers demonstrated efficient mRNA‑lipid nanoparticle (LNP) delivery to these cells (LNP X), using Tat mRNA to awaken latent virus ex vivo—without broad cell activation. This approach also carried CRISPR activation machinery, opening precision “kick‑and‑kill” strategies that could pair with immune clearance. Prior studies showed synergy between Tat mRNA and classical latency‑reversing agents, reinforcing combination logic for future trials.15
Taken together, cure research is multi‑pronged—antibodies, cellular immunity, gene editing, and mRNA platforms—progressing from proof‑of‑concept toward cautious human experimentation. None is ready for routine care, but together they are shifting the horizon from indefinite daily management to durable remission strategies.
UNAIDS warns that 2025 funding cuts triggered a global prevention crisis, with projected 30–40% declines in external health assistance and widespread closures of community‑led services. Without rapid policy and financing fixes, models project millions of additional infections and deaths by 2029. United Nations reporting notes some countries are increasing domestic budgets, but these efforts cannot fully replace long‑standing international support; the call is for renewed solidarity to avoid reversing gains.2
On the innovation side, WHO and partners are using prequalification and collaborative registration procedures to accelerate access to long‑acting lenacapavir; foundations and procurement mechanisms are negotiating generic pathways aiming for ~$40/year pricing from 2027 across >100 countries—still with gaps for middle‑income settings. Similarly, CAB‑LA supply expansions target high‑burden regions through not‑for‑profit pricing and voluntary licenses.6
The science is ready to transform prevention and lighten treatment. Only fair pricing, sustainable financing, and community‑centered delivery can ensure these tools reach those most at risk.
The HIV response stands at a transformative juncture. By 2024, global metrics showed formidable progress in treatment coverage, viral suppression, and declines in infections and deaths. In 2025, science delivered twice‑yearly PrEP and promising six‑monthly treatment combinations, alongside immune‑guided remission signals from bNAbs, first‑in‑human CRISPR efforts, and mRNA‑based latency reversal. The arc is bending from daily management to long‑acting, closer‑to‑cure strategies.
But innovation will matter only if access keeps pace. The 2025 funding crisis is a stark reminder: without solidarity, sustainable financing, and fair pricing, lifesaving advances can remain out of reach, threatening to reverse hard‑won gains. The road to cure is not just scientific; it is political and operational.
For individuals, the next steps can be personal and practical: ask about long‑acting options, review clinic schedules, and weigh PrEP choices—including whether twice‑yearly lenacapavir or bi‑monthly cabotegravir align with your life. For health systems and funders, the mandate is clear: protect prevention, scale equitable access, and invest in the cure pipeline. If we meet those commitments, the coming decade can deliver lighter care today and durable remission tomorrow—a road to cure that is credible, inclusive, and within reach.
Reference
1. WHO. HIV statistics, globally and by WHO region, 2025. Available from: https://cdn.who.int/media/docs/default-source/hq-hiv-hepatitis-and-stis-library/who-ias-hiv-statistics_2025-new.pdf. [Accessed 25 November 2025]. 2. UNAIDS. UNAIDS releases its 2025 World AIDS Day report: Overcoming disruption, transforming the AIDS response. 25 November 2025. Available from: https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2025/november/wad-2025-report. [Accessed 3 December 2025]. 3. Xu JJ, et al. Chin Med J (Engl). 2021;134(23):2799–809. 4. Bekker L-G, et al. N Engl J Med. 2024;391(13):1179–92. 5. Kelley CF, et al. N Engl J Med. 2025;392(13):1261–76. 6. PrEP Watch. Guidelines on Lenacapavir for HIV Prevention and Testing Strategies for Long-Acting Injectable Pre-Exposure Prophylaxis. 18 July 2025. Available from: https://www.prepwatch.org/resources/guidelines-on-lenacapavir-for-hiv-prevention-and-testing-strategies-for-long-acting-injectable-pre-exposure-prophylaxis/. [Accessed 25 November 2025]. 7. Adepoju VA, et al. Int J Equity Health. 2025;24(1):270. 8. Sah S, et al. J Int Assoc Provid AIDS Care. 2025 Nov 11;24:23259582251390622. 9. Zhang J, et al. Lancet HIV. 2022;9(4):e254–e268. 10. Jamieson L, et al. Lancet HIV. 2022;9(12):e857–e867. 11. Pinto RM, et al. J Assoc Nurses AIDS Care. 2023;34(2):216–20. 12. Eron JJ, et al. J Infect Dis. 2025 ;231(6):1440–4. 13. Liu Y, et al. Emerg Microbes Infect. 2020;9(1):194–206. 14. Hussein M, et al. Int J Mol Sci. 2023;24(2):1563. 15. Cevaal PM, et al. Nat Commun. 2025;16(1):4979.