Prostate cancer (PCa) is one of the most common malignancies in men worldwide, with incidence projected to rise from 1.4 million in 2020 to 2.9 million by 2040 due to aging populations and increased life expectancy1. In Hong Kong, PCa was the third most common cancer in men in 2022, accounting for 16% of male cancer diagnoses with 2,758 new cases, and the fourth leading cause of male cancer deaths with 519 deaths2. From 2012 to 2022, new cases increased by 69%2. In China, the burden is also growing rapidly, with 134,200 new cases and 47,500 deaths reported in 2022, and annual increases in age-standardized incidence and mortality of 7.0% and 4.1%, respectively3.
Despite advances such as multi-parametric MRI, PCa screening and diagnosis remain challenged by overdiagnosis and overtreatment, with estimates ranging from 1.7% to 67% depending on population and method4. Variability in practice, inter-observer differences, and inconsistent staging contribute to unnecessary biopsies, treatment-related morbidity (e.g. erectile dysfunction), reduced quality of life, and increased healthcare costs5. This underscores the need to refine and standardize diagnostic pathways and enable more personalized, risk-adapted treatment.
Recent growth in large-scale clinical, imaging, and molecular data, along with advances in artificial intelligence (AI) such as computer vision and large language models (LLMs), offers a path to transform precision oncology1. For PCa, a disease marked by heterogeneity, AI and meta-data analytics, analysis of information that describes the data, hold particular promise to improve diagnostic accuracy and support truly personalized treatment strategies.
AI is advancing the diagnosis of localized PCa and supporting more precise risk assessment. Deep learning (DL) methods applied to imaging and pathology can detect complex, subtle features in high-resolution scans that might be missed by the human eye, helping radiologists and pathologists deliver more accurate diagnoses and consistent risk stratification.
AI and advanced analytics are improving PCa imaging by enhancing detection accuracy, risk stratification, and treatment planning. There is increasing interest in using MRI alone to identify clinically significant PCa (csPCa), driving development of AI-based methods for automated segmentation and lesion detection1. These methods leverage multiparametric MRI (mpMRI), which combines T2-weighted imaging for detailed anatomy, diffusion-weighted imaging for cellular density and tumor aggressiveness, and dynamic contrast-enhanced imaging to assess vascularity1.
The Prostate Imaging Reporting and Data System (PI-RADS) is the global standard for interpreting prostate MRI, scoring lesions from 1–2 (low likelihood of csPCa) to 4–5 (high likelihood), with PI-RADS 3 representing an equivocal category that creates diagnostic challenges6. Recent studies show machine learning models can match or surpass PI-RADS in distinguishing csPCa from clinically insignificant disease7. DL models also help standardize PI-RADS scoring, reducing interobserver variability that leads to false positives and unnecessary biopsies, which carry both procedural and psychological burden1. For example, ResNet34, a popular deep learning model, has shown promise in automating PI-RADS classification on segmented lesions (Figure. 1)6.
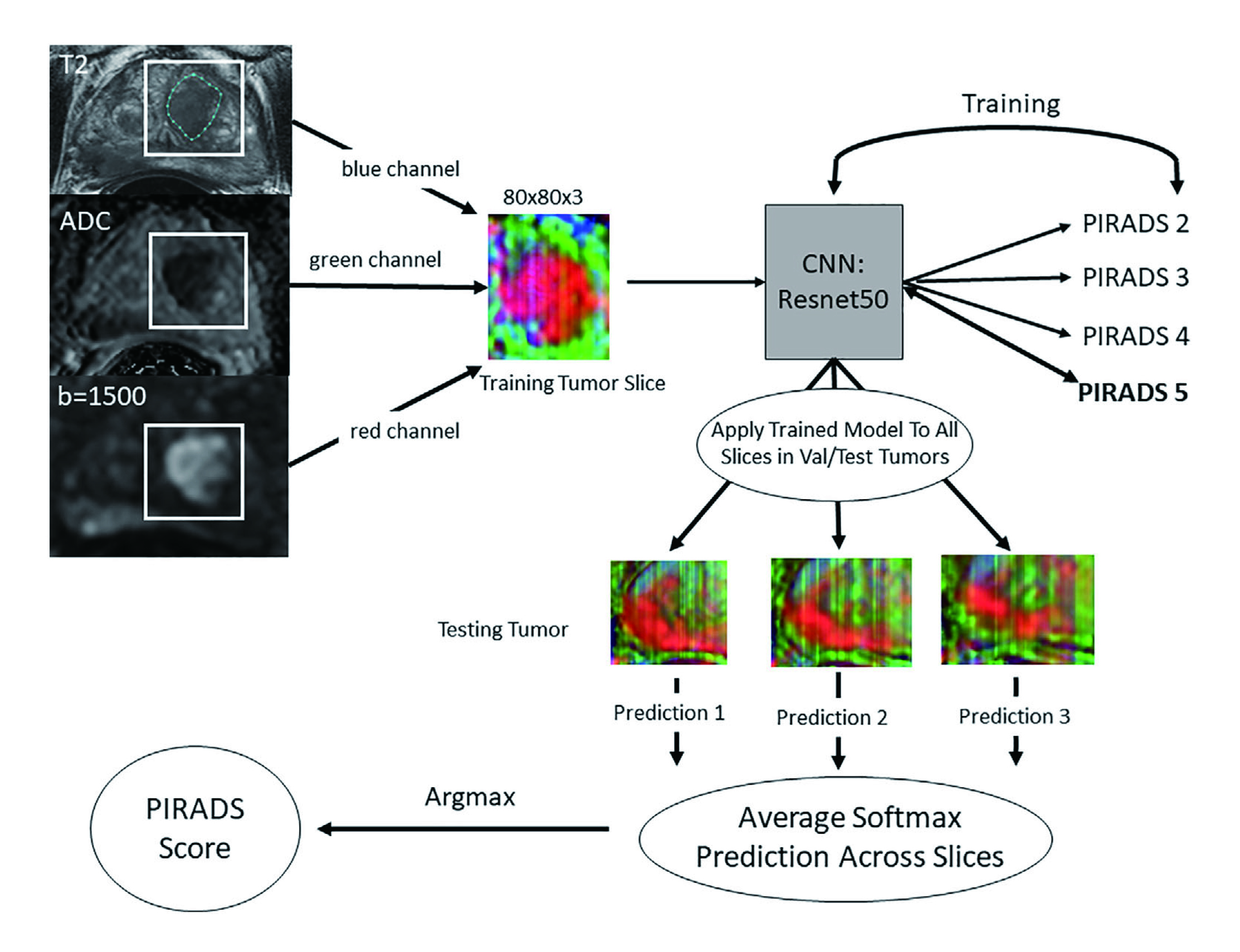
Figure 1: Workflow of ResNet34 for data processing, model training, and per-lesion model application6
AI applications have also been explored for predicting Gleason grades, which is a key prognostic marker for localized PCa, from MRI images (Figure. 2); although current approaches face challenges in generalizability due to their reliance on histopathology for training1,8. Saha et al. demonstrated an autonomous DL model based on U-Net architecture achieving an area under the curve (AUC) of 0.88 for detecting csPCa on MRI9.
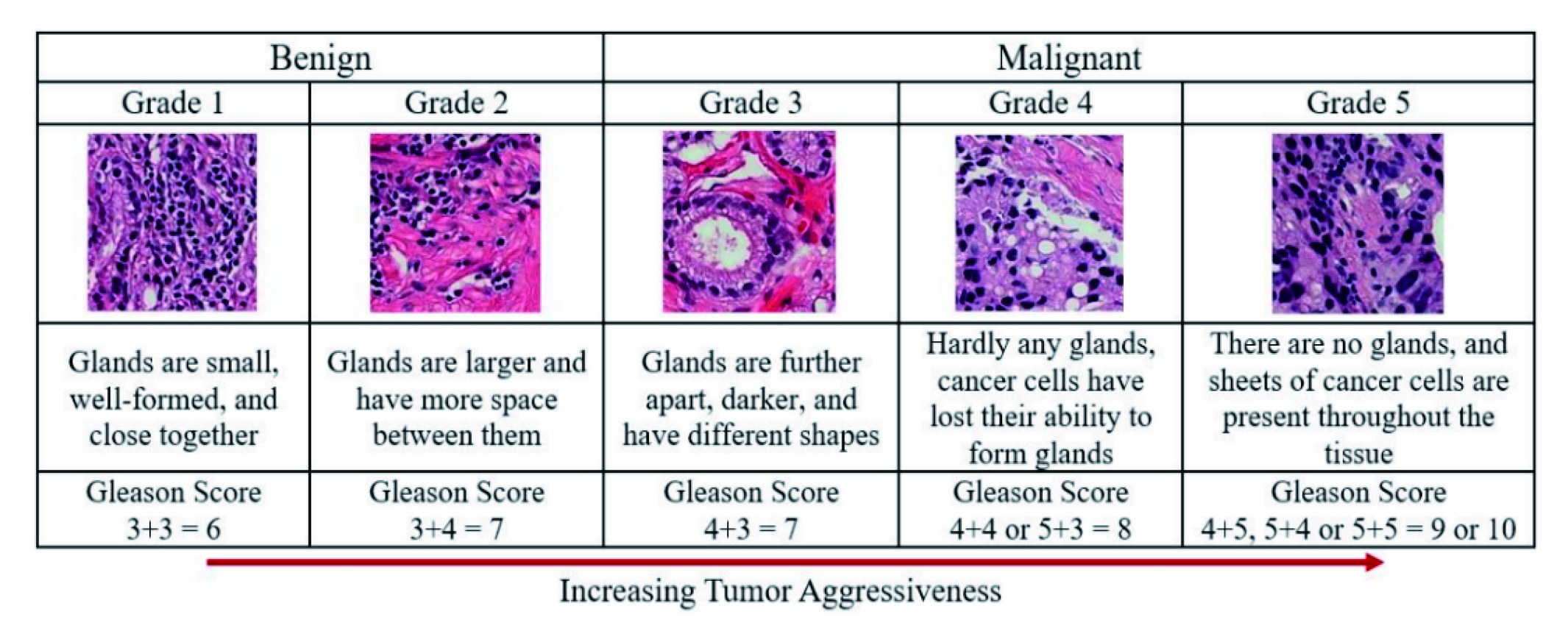
Figure 2: Gleason scores and prostate cancer grading system8
Beyond segmentation and scoring, AI offers broader benefits in imaging analysis. Radiomics approaches extract quantitative biomarkers such as tumor size, shape, texture, and intensity to support objective diagnosis and treatment planning7. Additionally, AI has also enabled converting 2D histopathology slides into 3D models to improve visualization and risk assessment7. DL methods bypass manual feature selection, instead modeling complex relationships between imaging and clinical outcomes directly, to enable automated segmentation, detection, and classification7. These tools help clinicians deliver personalized, data-driven treatment strategies tailored to individual risk profiles7. Importantly, several AI-powered software solutions with FDA clearance are now available to assist radiologists, reflecting growing clinical acceptance1. As AI continues to integrate large-scale imaging and clinical datasets, it has substantial potential to standardize diagnostic pathways, reduce unnecessary interventions, and improve patient outcomes through more precise, individualized care1,7.
The standard for PCa diagnosis relies on examining biopsy specimens, with pathologists manually reviewing slides to detect cancer and assign Gleason grades, which are critical prognostic markers guiding treatment decisions1. However, this manual process can be time-consuming and is subject to interobserver variability1,7. Advances in AI have led to rapid growth in automated cancer detection using digitized whole-slide images with convolutional neural networks (CNN) frequently applied for this task1. Multiple studies have shown high slide-level accuracy, with AUC values exceeding 0.91. For example, Paige Prostate is the first FDA-approved AI system for automated detection in core needle biopsies, achieving sensitivity above 94% and specificity over 93% in validation studies1.
Beyond detection, AI has been explored for automated Gleason grading to improve precision and reduce variability, achieving results comparable to or exceeding expert pathologists1,7. Nagpal et al. developed a DL system with diagnostic accuracy of 0.70 and high concordance among pathologists1. Bulten et al. trained a CNN that reached an AUC of 0.99 distinguishing benign from malignant samples in a test set of 535 biopsies, outperforming less-experienced pathologists while matching senior experts7. Other models such as Galen Prostate achieved an AUC of 0.941 distinguishing low- from high-grade cancer1, while DeepDX Prostate reported high diagnostic concordance with a quadratic kappa of 0.9071. Integrating AI has improved workflow efficiency, reducing analysis time by approximately 65.5% while detecting PCa cases that were missed by multiple pathologists7.
Personalized treatment in PCa has emerged as a critical advance in modern oncology, shifting care away from a one-size-fits-all approach toward strategies tailored to each patient's unique disease profile and personal goals. This approach considers genetics and genomic mutations, tumor location, volume, aggressiveness, patient age, comorbidities, and molecular or biomarker profiles to guide prevention, diagnosis, and treatment strategies, aiming to maximize effectiveness while minimizing side effects. Current practices include genetic and genomic profiling to detect DNA repair gene mutations like BRCA1/2 or ATM, supporting targeted therapy selection in advanced or metastatic disease10; the use of poly (ADP-ribose) polymerase PARP inhibitors such as olaparib for patients with actionable mutations10; biomarker-guided decisions using tests like SelectMDx to identify patients who need more aggressive treatment versus those suitable for surveillance11; and risk-adapted therapy that aligns treatment intensity and modality with tumor characteristics and patient preferences12. As AI technologies and the availability of large-scale meta-data continue to advance, there is significant potential to further refine and improve the precision of personalized treatment strategies in PCa, ensuring care is even more closely tailored to individual risk and biology.
LLMs can efficiently extract structured details from electronic health records, pathology reports, and clinical notes to support real-time clinical decision-making. Beyond text extraction, AI models using computer vision are being developed to quantify disease volume more accurately than conventional lesion-count methods, while multimodal frameworks aim to integrate imaging, genomics, patient demographics, and clinical text for more nuanced risk assessment in metastatic PCa1. For instance, a validated multimodal digital pathology model using data from the phase III STAMPEDE trial stratified patients by PCa–specific mortality risk1. In addition, Esteva et al. developed a multimodal deep learning system that integrates clinical data with unannotated biopsy histopathology, trained on five phase III trials with 5,654 of 7,764 patients (71%) and a median follow-up of 11.4 years. The model outperformed the National Comprehensive Cancer Network (NCCN) risk-stratification tool across all endpoints, achieving 9.2% to 14.6% relative improvement in discriminatory performance13. Unlike traditional methods requiring labor-intensive region-level annotations, this system uses self-supervised learning to extract features from histopathology slides, lowering barriers to deployment in new clinics with only digital scanners and internet access13. AI for Prognosis, Recurrence Prediction, and Clinical Decision Support AI and machine learning are advancing PCa prognosis by enabling personalized predictions that inform treatment selection. Models trained on large datasets predict survival and mortality with high accuracy, such as that Bibault et al. developed an algorithm achieving 0.98 accuracy using 30 clinical variables7. Koo et al. constructed a neural network tool that uses data from over 7,200 patients to provide individualized 5- and 10-year survival estimates7. For recurrence prediction after radical prostatectomy, Tan et al. established an AI model that achieved an AUC of 0.894 at 5 years, while Huynh et al. reported an AUC of 0.89 using MRI radiomics, both surpassing traditional nomograms7. Web-based tools like UCSF-CAPRA and askMUSIC further help clinicians and patients visualize personalized recurrence risks and treatment choices7.
AI and machine learning are advancing PCa prognosis by enabling personalized predictions that inform treatment selection. Models trained on large datasets predict survival and mortality with high accuracy, such as that Bibault et al. developed an algorithm achieving 0.98 accuracy using 30 clinical variables7. Koo et al. constructed a neural network tool that uses data from over 7,200 patients to provide individualized 5- and 10-year survival estimates7. For recurrence prediction after radical prostatectomy, Tan et al. established an AI model that achieved an AUC of 0.894 at 5 years, while Huynh et al. reported an AUC of 0.89 using MRI radiomics, both surpassing traditional nomograms7. Web-based tools like UCSF-CAPRA and askMUSIC further help clinicians and patients visualize personalized recurrence risks and treatment choices7.
The integration of artificial intelligence and meta-data analytics holds transformative potential for PCa care by enabling more accurate, individualized diagnosis and treatment selection. By combining clinical, imaging, genomic, and pathology data at scale, AI models deliver precise risk stratification and prognosis predictions that account for patient-specific factors often missed by traditional tools. This level of personalization helps clinicians distinguish between indolent and aggressive disease, reducing unnecessary biopsies, overtreatment, and their associated side effects, while ensuring timely, appropriate intervention for high-risk patients. Furthermore, AI-powered decision support systems and web tools improve clinician-patient communication, supporting shared, evidence-based treatment choices. As digital pathology and large-scale data resources continue to expand, AI-driven approaches promise to democratize access to precision oncology worldwide, lowering barriers to adoption and offering a practical path toward delivering safer, more effective, and truly patient-centered PCa care.
References
1. Riaz IB, Harmon S, Chen Z, Naqvi SAA, Cheng L. Applications of artificial intelligence in prostate cancer care: a path to enhanced efficiency and outcomes. Am Soc Clin Oncol Educ Book. 2024 Jun;44(3):e438516. doi:10.1200/EDBK_438516. PMID: 38935882. 2. Hong Kong Cancer Expert Working Group on Cancer Prevention and Screening. Prostate Cancer [Internet]. Hong Kong: Cancer Expert Working Group; [cited 2025 Jul 9]. Available from: https://www.cancer.gov.hk/en/hong_kong_cancer/common_cancers_in_hong_kong/prostate_cancer.html 3. Wang Z, Xu W, Wan F, Tian X, Anwaier A, Ye S, Zhou S, Zhang H, Qin X, Ye D. Prostate cancer in China: epidemiological trends, genomic insights, and future directions for optimized management. J Natl Cancer Cent. 2025. 4. Loeb S, Bjurlin MA, Nicholson J, Tammela TL, Penson DF, Carter HB, Carroll P, Etzioni R. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014 Jun;65(6):1046-55. doi:10.1016/j.eururo.2013.12.062. PMID: 24439788; PMCID: PMC4113338. 5. Dushimova Z, Iztleuov Y, Chingayeva G, Shepetov A, Mustapayeva N, Shatkovskaya O, Pashimov M, Saliev T. Overdiagnosis and overtreatment in prostate cancer. Diseases. 2025 May 24;13(6):167. doi:10.3390/diseases13060167. PMID: 40558578; PMCID: PMC12191725. 6. Sanford T, Harmon SA, Turkbey EB, Kesani D, Tuncer S, Madariaga M, Yang C, Sackett J, Mehralivand S, Yan P, Xu S, Wood BJ, Merino MJ, Pinto PA, Choyke PL, Turkbey B. Deep-learning-based artificial intelligence for PI-RADS classification to assist multiparametric prostate MRI interpretation: a development study. J Magn Reson Imaging. 2020 Nov;52(5):1499-1507. doi:10.1002/jmri.27204. PMID: 32478955; PMCID: PMC8942293. 7. Arita Y, Roest C, Kwee TC, Paudyal R, Lema-Dopico A, Fransen S, Hirahara D, Takaya E, Ueda R, Ruby L, Nissan N, Schwartz LH, Shukla-Dave A, Akin O. Advancements in artificial intelligence for prostate cancer: optimizing diagnosis, treatment, and prognostic assessment. Asian J Urol. 2025. 8. Bhattacharjee S, Kim CH, Park HG, Prakash D, Madusanka N, Cho NH, Choi HK. Multi-features classification of prostate carcinoma observed in histological sections: analysis of wavelet-based texture and colour features. Cancers (Basel). 2019 Dec 4;11(12):1937. doi:10.3390/cancers11121937. PMID: 31817111; PMCID: PMC6966617. 9. Saha A, Hosseinzadeh M, Huisman H. End-to-end prostate cancer detection in bpMRI via 3D CNNs: effects of attention mechanisms, clinical priori and decoupled false positive reduction. Med Image Anal. 2021 Oct;73:102155. doi:10.1016/j.media.2021.102155. PMID: 34245943. 10. Fenton SE, et al. Advancing prostate cancer care: treatment approaches to precision medicine, biomarker innovations, and equitable access. Am Soc Clin Oncol Educ Book. 2024;44:e433138. doi:10.1200/EDBK_433138. 11. Visser WCH, de Jong H, Steyaert S, Melchers WJG, Mulders PFA, Schalken JA. Clinical use of the mRNA urinary biomarker SelectMDx test for prostate cancer. Prostate Cancer Prostatic Dis. 2022 Sep;25(3):583-589. doi:10.1038/s41391-022-00562-1. PMID: 35810263; PMCID: PMC9385481. 12. Hayden AJ, Catton C, Pickles T. Radiation therapy in prostate cancer: a risk-adapted strategy. Curr Oncol. 2010 Sep;17 Suppl 2:S18-24. doi:10.3747/co.v17i0.704. PMID: 20882127; PMCID: PMC2935704. 13. Esteva A, Feng J, van der Wal D, et al. Prostate cancer therapy personalization via multi-modal deep learning on randomized phase III clinical trials. NPJ Digit Med. 2022;5:71. doi:10.1038/s41746-022-00613-w.