Dr. Leung Ka Chun
Resident Specialist, Tuen Mun Hospital, New Territories West Cluster, Hospital Authority

Dr. Cheung Cheuk Yiu Ben
Associate Consultant, Yan Chai Hospital, Kowloon West Cluster, Hospital Authority

Dr. Lau Lik Fung Sam
Associate Consultant, Prince of Wales Hospital, New Territories East Cluster, Hospital Authority

Dr. Lam Chi Kwan Darwin
Associate Consultant, United Christian Hospital, Kowloon East Cluster, Hospital Authority

Dr. Wong Chi Kwan
Consultant, Pamela Youde Nethersole Eastern Hospital, Hong Kong East Cluster, Hospital Authority

Dr. Chan Koon Ming
Associate Consultant, Queen Elizabeth Hospital, Kowloon Central Cluster, Hospital Authority
Cardiovascular-kidney-metabolic (CKM) syndrome is a health disorder attributable to the connections among cardiovascular disease (CVD), chronic kidney disease (CKD), and metabolic diseases, such as diabetes mellitus (DM) and obesity. Given that each of the 3 disorders can lead to or worsen one another, the management of CKM syndrome is clinically challenging1. Accordingly, screening for CKM risk factors and timely intervention are crucial for preventing CKM syndrome and hence optimising patient outcomes. As CKM syndrome involves multiple organ systems, interdisciplinary cooperation among various specialties is essential. In particular, the primary care provided by general practitioners (GPs) plays a pivotal role in managing CKM syndrome. In the recent lecture series jointly organised by the Hong Kong Society of Nephrology (HKSN), the Hong Kong Kidney Foundation (HKKF), and the Hong Kong Association of Renal Nurses (HKARN), a panel of 6 local nephrologists was invited to discuss the pathophysiology of CKM and share their opinions to tackle this disorder.
CKM syndrome is a novel construct emphasising the pathophysiological interplay of CVD, CKD, and metabolic derangements, including those at risk for CVD and those with existing CVD. In addition to highlighting the risk of comorbid conditions, addressing the CKM concept enables the implementation of a holistic framework that incorporates screening, staging, and management for the early identification of potential CKM-related events2. The overarching principle of CKM care is to adopt a multidisciplinary approach which seeks to prevent fragmented care.
Although the public awareness of CKM syndrome is low, CKM-related events are very common. The serial cross-sectional cohort study (2023) of 11,607 adults in the United States (US) suggested that >1 in 4 participants aged ≥65 years had ≥1 cardiac, renal, and metabolic condition, whereas cardiac, renal, and metabolic multimorbidity was observed in 8% of participants (Figure 1). Remarkably, a significant increase in prevalence was observed from 5.3% in 1999-2000 to 8.0% in 2017-20203.
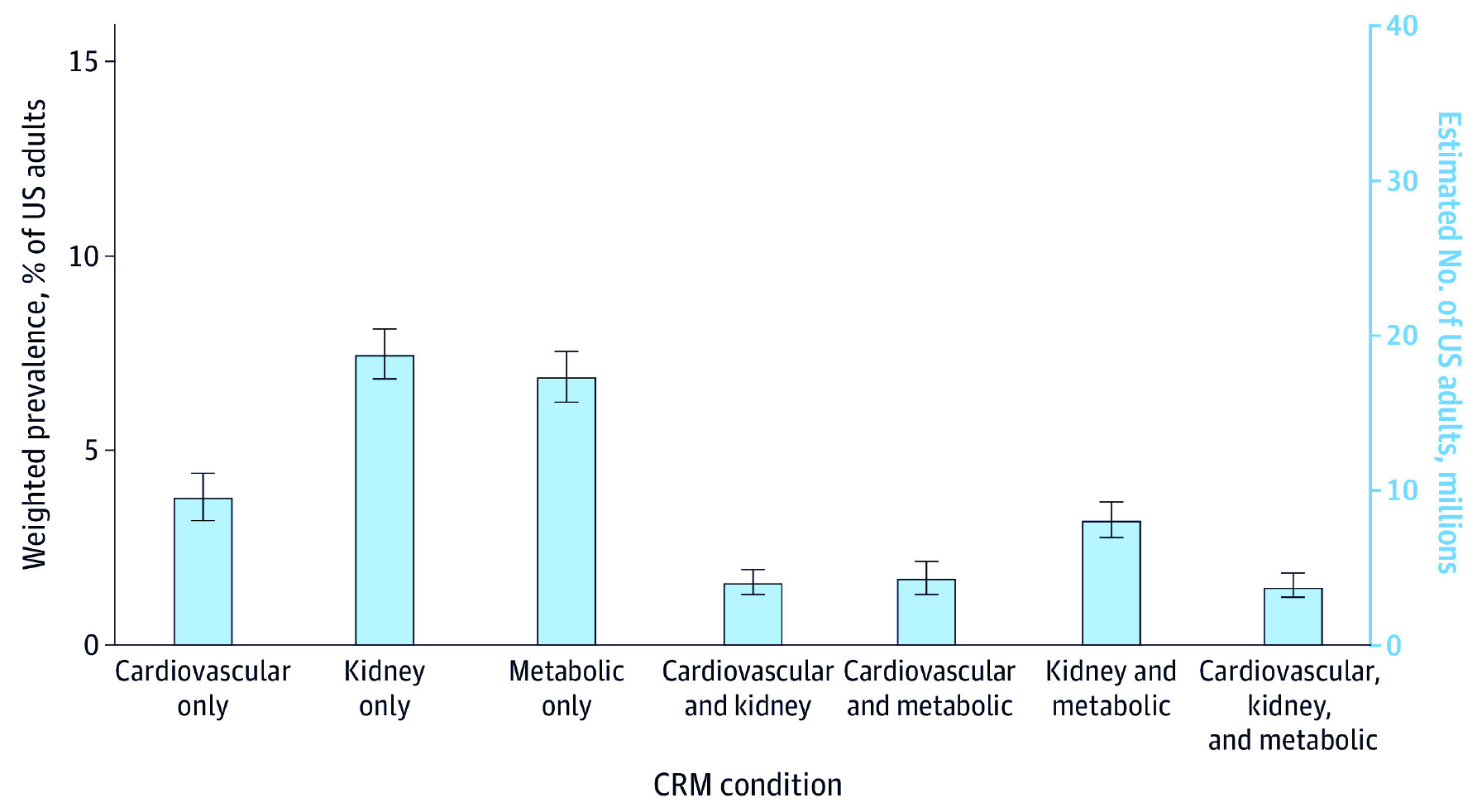 Figure 1. Prevalence and overlap of CKM conditions in US adults3
Figure 1. Prevalence and overlap of CKM conditions in US adults3
A nationwide cohort study involving 877,537 participants by Kim et al. (2025) indicated that 15.3% of the participants experienced CKM stage progression. Compared to individuals who maintained the same stage of CKM, those with progression of CKM stages had a higher risk of composite outcome of all-cause death, heart failure (HF), stroke, and myocardial infarction (MI)4. Provided the increased risk of mortality and comorbidities, CKM conditions are expected to escalate healthcare costs.
It has been generally recognised that CKM syndrome is a progressive condition. Accordingly, the American Heart Association (AHA) addressed the CKM staging to highlight the stepwise increase in absolute CVD risk. Essentially, the staging model underscores the importance of early detection of CKM-related changes and guides intensified therapies for each CKM stage. At stage 0, no CKM risk factor is observed. Stage 1 indicates excess or dysfunctional adiposity but no evidence of subclinical or clinical CKD or CVD. Stage 2 refers to the occurrence of established metabolic risk factors or CKD, whereas stage 3 suggests a subclinical CVD in CKM syndrome and a very high risk of CKD. At stage 4, clinical CVD occurs without (stage 4a) or with (stage 4b) kidney failure (Figure 2)5. The panel emphasised that implementing effective preventive measures, particularly at stages 0 to 2, helps reduce, or even avoid, disease progression to later CKM stages.
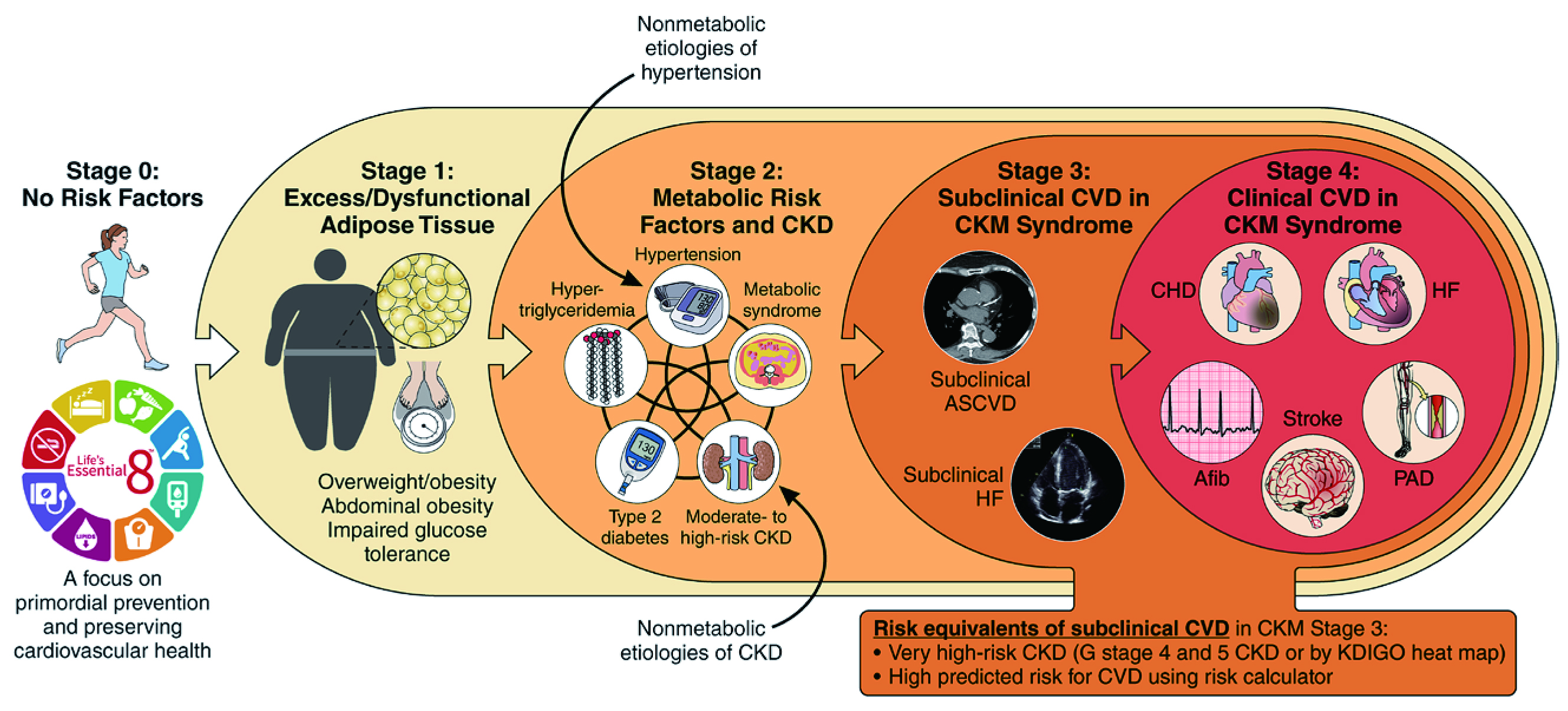 Figure 2. Stages of CKM syndrome5
Figure 2. Stages of CKM syndrome5
The CKM health framework prioritises identifying and treating CKM risk factors during the preclinical phase to prevent CV complications and kidney failure. In this regard, absolute risk assessment based on an individual’s risk factors and clinical signs is crucial for reflecting one’s risk of disease events. For instance, the PREVENT equations proposed by the AHA (2023), which consider factors along the 3 axes of CKM syndrome, such as estimated glomerular filtration rate (eGFR), as predictors, enable the estimation of 10- and 30-year risk for total CVD6.
In the context of CKD, the Kidney Disease: Improving Global Outcomes (KDIGO) 2024 Clinical Guidelines recommended that the risk of CKD should be estimated based on both eGFR and albuminuria (Figure 3). For instance, an eGFR 45-59ml/min/1.73 m2 indicates mildly to moderately decreased kidney function and corresponds to stage 3a CKD, whereas a moderately increased albuminuria is indicated by the level of 30-299 mg/g. The co-existence of moderately decreased kidney function and moderately increased albuminuria suggests a high risk of disease progression, and monitoring twice a year is recommended7. Notably, the panel reminded that the diagnosis of CKD is documented based on 2 separate measurements, at least 3 months apart.
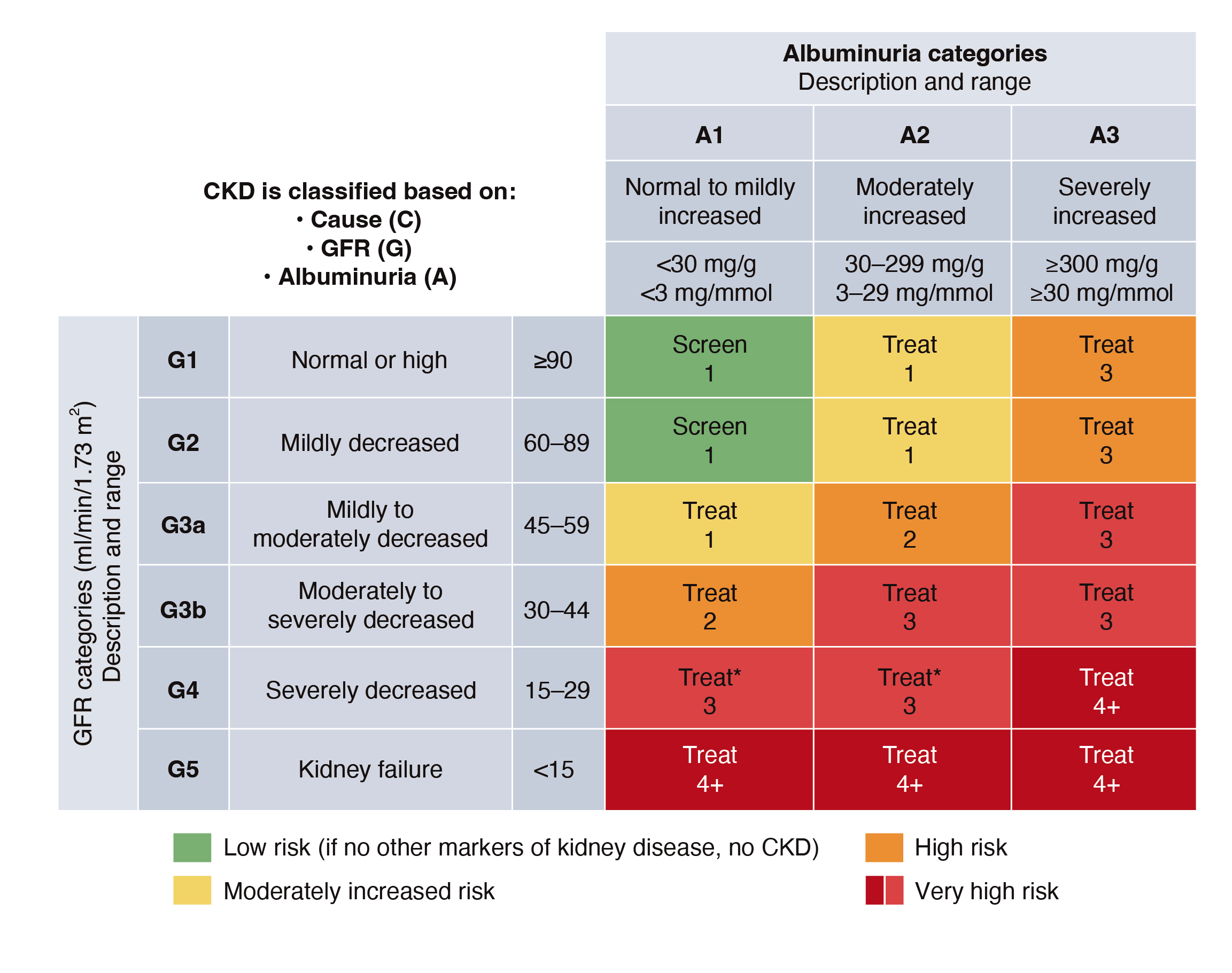
Figure 3. Albuminuria and GFR grid reflect the risk of CKD progression7
The essence of CKM screening is to identify individuals at risk for suboptimal CKM health, facilitating timely intervention and preventing complications. Given that most individuals are asymptomatic at early CKM stages, primary care doctors play a central role in identifying these cases and ensuring regular follow-up. For patients exhibiting CKD risk factors, including hypertension, DM, and CVD, the measurements of eGFR and albuminuria are recommended for early diagnosis of CKD.
To facilitate the early identification of CKM cases, the panel advocates for a universal approach to CKM screening for all asymptomatic adults, which includes the annual measurement of BMI (body mass index), waist circumference, blood pressure, lipid levels, glycaemic status, urinalysis, and eGFR. Besides, the stage-dependent approach, which specifies screening intervals, is recommended as well (Table 1).
Table 1. Screening interval as per the stage-dependent approach
|
CKM Stage |
Clinical Presentations |
Screening Interval |
|
Stage 0 |
Healthy |
Every 3-5 years |
|
Stage 1 |
Overweight/obese or prediabetes |
Every 2-3 years |
|
Stage 2 |
DM, hypertension, or hypertriglyceridemia |
Annually |
The management of CKM syndrome is initiated at stage 0, even though no CKM risk factors are observed. The interventions at CKM stages 0 and 1 focus on lifestyle modification and weight management, with the goal of preventing metabolic risk factors and CKD5. At stage CKM stage 2, the CKM risk factors are established. In addition to more intensive lifestyle intervention targeting multifactorial risk control, pharmacotherapies for comprehensive control of residually uncontrolled metabolic syndrome components are recommended5.
Remarkably, the first consensus recommendation on the management of CKD in Hong Kong has been published recently. In addition to CKD, the consensus has taken the components of CKM syndrome into account. Particularly, the consensus recommended that persons with hypertension, DM, or CVD should be screened for CKD. Besides, the consensus recommended to monitor the eGFR and albuminuria of patients with early CKD stages at least twice annually and more often in those with a higher risk of progression8.
According to the local consensus on CKD management, first-line treatment with the maximum tolerated dose of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (ACEi/ARB) should be initiated for patients with DM and hypertension or albuminuria for renal protection and blood pressure control. Besides, ACEi/ARB is also recommended in non-diabetic CKD patients with hypertension and a urine albumin-creatinine ratio of ≥200 mg/g for blood pressure control and renal protection8.
In patients with type 2 DM and CKD (eGFR≥20ml/min/1.73m2), a sodium-glucose cotransporter-2 inhibitor (SGLT2i) can be initiated as first-line therapy for glycaemic control, renal and cardiovascular protection. Moreover, SGLT2i can also be used in non-diabetic CKD patients with an eGFR of ≥20 mL/min/1.73 m2 and a urine albumin-creatinine ratio of ≥200 mg/g for renal and cardiovascular protection8.
Renin-angiotensin system (RAS) blockade, SGLT2i, non-steroidal mineralocorticoid receptor antagonists (ns-MRA), and glucagon-like receptor 1 receptor agonists (GLP-1RAs) are the 4 pillars of pharmacotherapies in CKM management. In addition to pharmacotherapies, the panel emphasised that lifestyle modifications, lipid control, as well as blood pressure and glycaemic control also play vital roles in controlling CKM syndrome.
Apart from controlling blood pressure, the inhibition of RAS by ACEi/ARB has been proven to be effective in reducing intraglomerular pressure by preferentially dilating the efferent arteriole9. For instance, the network meta-analysis by Palmer et al. (2015) suggested that ACEi/ARB were the most effective strategies to reduce the risk of end-stage kidney disease (ESKD) among patients with type 2 DM and CKD compared to other blood pressure-lowering agents10.
Regarding the issue of hyperkalaemia upon ACEi/ARB treatment, the panel opined that there should not be a simple cut-off for discontinuing treatment at 5.0 mmol/L. Instead, monitoring serum creatinine and potassium within 2-4 weeks after starting or changing the dose of ACEi/ARB is recommended to confirm the occurrence of hyperkalaemia. In cases where hyperkalaemia occurs, optimising the patient’s diet to reduce potassium intake and considering medications, such as diuretics and sodium bicarbonate, are advisable. Reducing the dose or stopping ACEi/ARB may be considered if mitigation strategies are ineffective7.
In the case of advanced CKD, Bhandari et al. (2022) reported that discontinuation of RAS inhibitors was not associated with a significant difference in the long-term rate of decrease in the eGFR11. Nevertheless, there were numerically greater number of CV events in patients who discontinued SGLT2i. Therefore, the panel recommended continuation of RAS inhibitors for patients even in advanced CKD (eGFR <30ml/min/1.73m2) to reduce the risks of CV complications.
As per the KDIGO 2024 Guidelines, an individualised HbA1c target ranging from <6.5% to <8.0% is recommended in patients with DM and CKD not treated with dialysis7. Of note, SGLT2i facilitates glycaemic control by increasing urinary glucose excretion. Furthermore, SGLT2i also reduces intraglomerular pressure and appears to attenuate the inflammatory and fibrotic responses of human proximal tubular epithelial cells to high glucose9.
The cardiorenal protective effects of SGLT2i have been well established. For instance, a meta-analysis by McGuire et al. (2021) indicated that SGLT2i was associated with a reduced risk of major adverse cardiovascular events, hospitalisation for heart failure, or cardiovascular death (Figure 4A), and kidney outcomes (Figure 4B)12.
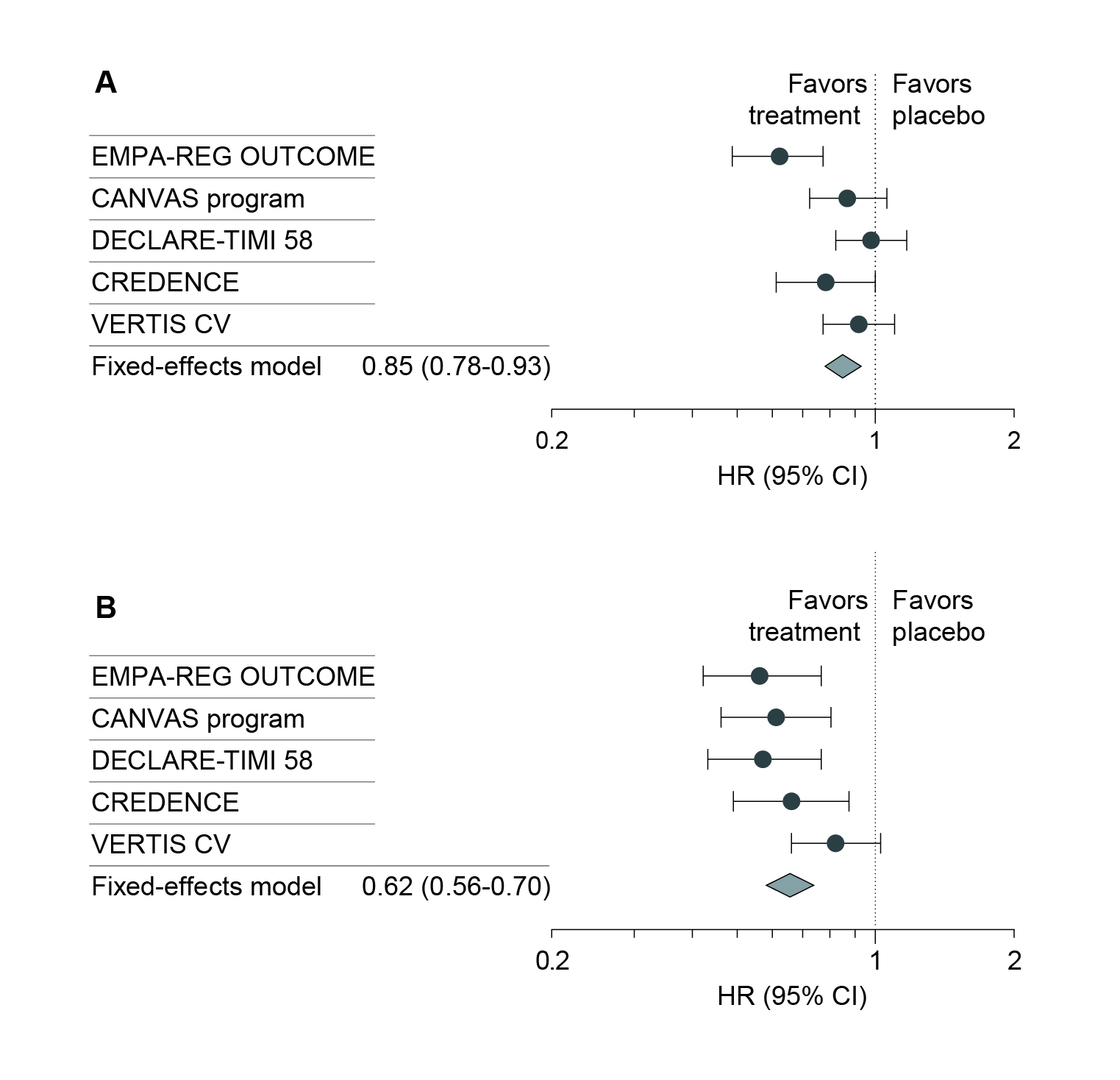
Figure 4. Effects of SGLT2i on A) overall cardiovascular death, and B) overall kidney outcomes12
Given the clinical benefits of SGLT2i in patients with eGFR≥20ml/min/1.73m2 are widely accepted, the panel advised that SGLT2i should be continued when eGFR drops below 20ml/min/1.73m2. Interestingly, an estimate of up to 16 years delay to kidney replacement therapy (KRT) could be achieved for commencing SGLT2i treatment when eGFR=60ml/min/1.73m2, whereas a delay of only 6.4 years would be yielded for commencing SGLT2i when eGFR=30ml/min/1.73m2 (Figure 5). Essentially, the delay to KRT implies the saving of time and expenses associated with haemodialysis, while preserving the patient’s quality of life.
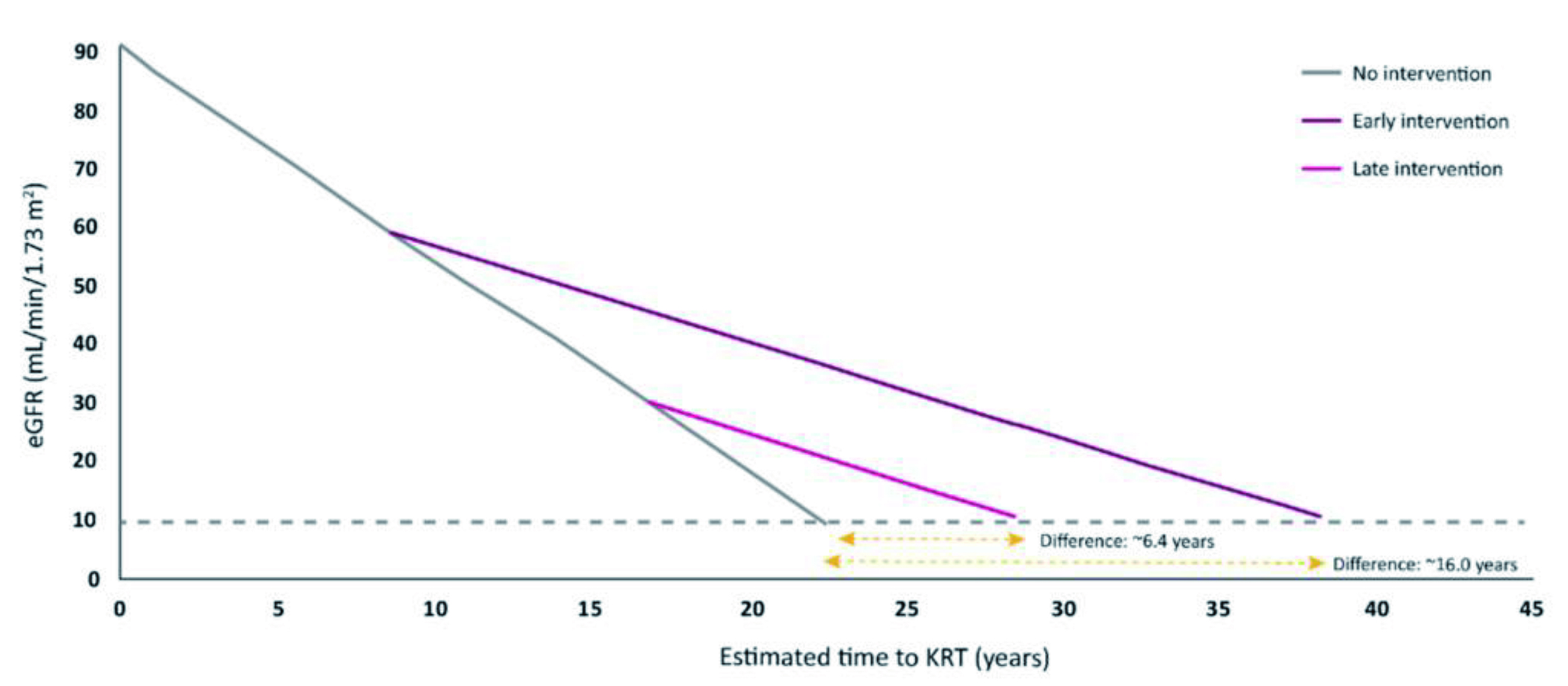
Figure 5. Estimated delay of KRT by early prescription of SGLT2i
Mineralocorticoid receptor (MR) activation is closely related to the development of inflammation and fibrosis in the heart, kidneys, and vasculature. Thus, inhibiting MR with ns-MRA offers a promising approach to control the progression of CKD and improve cardiovascular morbidity and mortality13. According to the FIDELITY study, finerenone, an ns-MRA, was associated with reduced risks of composite cardiovascular outcomes (hazard ratio [HR]: 0.86, p=0.0018) and kidney disease progression (HR: 0.77, p=0.0002, Figure 6), when compared to placebo among patients with diabetic kidney disease (DKD) across a range of GFR14.
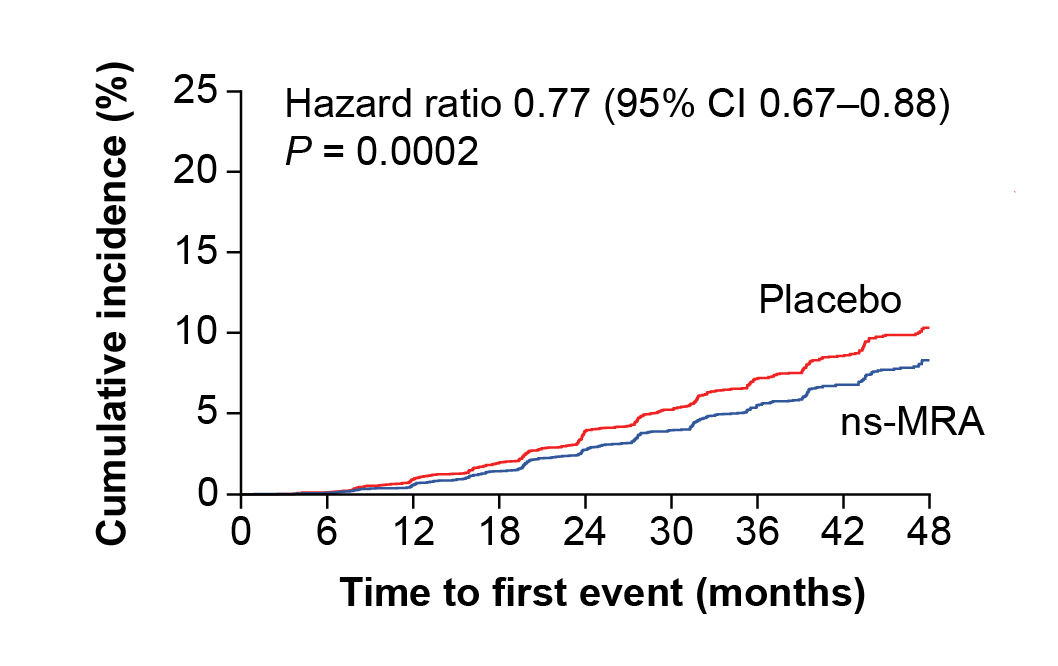
Figure 6. Composite kidney outcomes on patients treated with ns-MRA or placebo14
Of note, hyperkalaemia is a clinical concern associated with finerenone. Nonetheless, the pooled results of the FIDELITY study revealed that <2.0% of patients who received finerenone discontinued the treatment due to hyperkalaemia, and there was no fatal case reported14. Hence, the panel recommended the use of the maximum tolerated dose of finerenone if the serum potassium is ≤4.8mmol/L, while suspending finerenone is needed if the serum potassium level rises above 5.5mmol/L. Notably, the panel suggested that finerenone can be resumed if serum potassium level is reduced to ≤5.0mmol/L.
GLP-1RAs stimulate insulin release in response to glucose load through incretin release. By delaying gastric emptying and effects on the satiety centre in the brain, GLP-1RAs provoke weight loss15. Additionally, GLP-1RAs have been reported to provide cardiovascular and renal benefits. A meta-analysis by Sattar et al. (2021) revealed that GLP-1RAs reduced all-cause mortality (HR: 0.88, p=0.0001), hospital admission for heart failure (HHF, HR: 0.89, p=0.013), and composite kidney outcome (HR: 0.79, p<0.0001) than placebo, with no increase in risk of severe hypoglycaemia, retinopathy, or pancreatic adverse effects, among DM patients16. By virtue of the promising clinical benefits demonstrated in previous clinical trials, SGLT2is were included in the treatment recommendations of the US Food and Drug Administration (US FDA).
The panel recommended considering referral to nephrologists if persistent proteinuria (>1g/day) is observed despite optimisation of medications. Additionally, the involvement of nephrologists is advisable in the occurrence of nephrotic and nephritic symptoms, such as proteinuria and microscopic haematuria, as well as any unexplained impaired renal function.
Clinical guidelines generally advocate lifestyle interventions as a core component in managing CKM syndrome at all stages. For first-line therapy, metformin and SGLT2i were recommended by the panel. Moreover, GLP-1RAs are prescribed if an individualised glycaemic target needs to be achieved. In cases where hypertension occurs, ACEi/ARB should be used. If the albumin-creatinine ratio (ACR) is ≥30mg/g and serum potassium is normal, ns-MRA should be considered for cardiovascular protection. Besides, statins would be needed to control hypercholesterolaemia.
To conclude, the panel emphasised that early recognition and treatment are key to better outcomes in managing CKM syndrome. Provided the asymptomatic nature of early CKM stages, screening for CKD and other CKM risk factors is of paramount importance. Collaborative community efforts by colleagues in primary care in screening and education are essential for identifying individuals at high risk for CKM syndrome and enhancing public awareness of the disease.
References
1. Ferdinand KC. An overview of cardiovascular-kidney-metabolic syndrome. Am J Manag Care 2024; 30: S181–8.
2. Lee CH, Tan G, Tang SCW, et al. Incorporating the cardiovascular-kidney-metabolic health framework into the local healthcare system: a position statement from the Hong Kong College of Physicians. Hong Kong Medical Journal 2025; 31: 58–64.
3. Ostrominski JW, Arnold S V., Butler J, et al. Prevalence and Overlap of Cardiac, Renal, and Metabolic Conditions in US Adults, 1999-2020. JAMA Cardiol 2023; 8: 1050–60.
4. Kim BS, Kim HJ, Kim H, Lee J, Shin JH, Sung KC. Longitudinal Changes in Cardiovascular-Kidney-Metabolic Syndrome Stages and Their Impact on Outcomes: A Nationwide Cohort Study. J Clin Med 2025; 14: 3888.
5. Ndumele CE, Rangaswami J, Chow SL, et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory from the American Heart Association. Circulation 2023; 148: 1606–35.
6. Khan SS, Coresh J, Pencina MJ, et al. Novel Prediction Equations for Absolute Risk Assessment of Total Cardiovascular Disease Incorporating Cardiovascular-Kidney-Metabolic Health: A Scientific Statement From the American Heart Association. Circulation 2023; 148: 1982–2004.
7. Stevens PE, Ahmed SB, Carrero JJ, et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int 2024; 105: S117–314.
8. Tang SCW, Ho KKL, Ko WWK, et al. Management of chronic kidney disease: a Hong Kong consensus recommendation. Hong Kong Medical Journal 2024; 30: 478–87.
9. Chan GCW, Tang SCW. Diabetic nephropathy: landmark clinical trials and tribulations. Nephrol Dial Transplant 2016; 31: 359–68.
10. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015; 385: 2047–56.
11. Bhandari S, Mehta S, Khwaja A, et al. Renin–Angiotensin System Inhibition in Advanced Chronic Kidney Disease. New England Journal of Medicine 2022; 387: 2021–32.
12. McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 Inhibitors With Cardiovascular and Kidney Outcomes in Patients With Type 2 Diabetes: A Meta-analysis. JAMA Cardiol 2021; 6: 148–58.
13. Georgianos PI, Agarwal R. Mineralocorticoid Receptor Antagonism in Chronic Kidney Disease. Kidney Int Rep 2021; 6: 2281.
14. Agarwal R, Filippatos G, Pitt B, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2022; 43: 474–84.
15. Agarwal R, Fouque D. The foundation and the four pillars of treatment for cardiorenal protection in people with chronic kidney disease and type 2 diabetes. Nephrol Dial Transplant 2023; 38: 253–7.
16. Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021; 9: 653–62.