The field of rheumatology has undergone transformative changes in 2025, driven by innovations in biologic therapies, precision medicine, digital health, and neuroimmune modulation. This article explores the most significant advances in the diagnosis and treatment of rheumatic diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and psoriatic arthritis (PsA). Key developments include the approval of novel biologics such as dapirolizumab pegol (DZP) and sonelokimab, the emergence of smart drug delivery systems, and the integration of artificial intelligence (AI) in clinical decision-making. These breakthroughs promise improved patient outcomes, reduced side effects, and more personalized care. The article concludes with a discussion on the implications of these advances for clinical practice and future research directions.
Rheumatology, the branch of medicine focused on autoimmune and inflammatory diseases affecting joints, muscles, and connective tissues, has seen a surge of innovation in 2025. With over 100 distinct conditions under its umbrella—including RA, SLE, and PsA—rheumatology has long been challenged by diagnostic complexity and treatment limitations.1, 2 However, recent scientific and technological advances are reshaping the landscape, offering new hope to millions of patients worldwide.
This article reviews the most impactful developments in rheumatology in 2025, highlighting new therapies, diagnostic tools, and care models that are redefining the standard of care.
One of the most promising therapies in 2025 is DZP, a novel, polyethylene glycol (PEG)-conjugated antigen-binding fragment (Fab'), lacking an Fc domain. DZP binds CD40L, blocking CD40-CD40L interactions and CD40 activation, and has broad modulatory effects on SLE immunopathology.3 The PHOENYCS GO phase 3 trial demonstrated that DZP resulted in significant improvement in disease activity at Week 48 vs placebo (40.9% vs 19.6%, nominal p<0.0001) and was generally well tolerated in patients with moderate-to-severe SLE (Figure 1).3
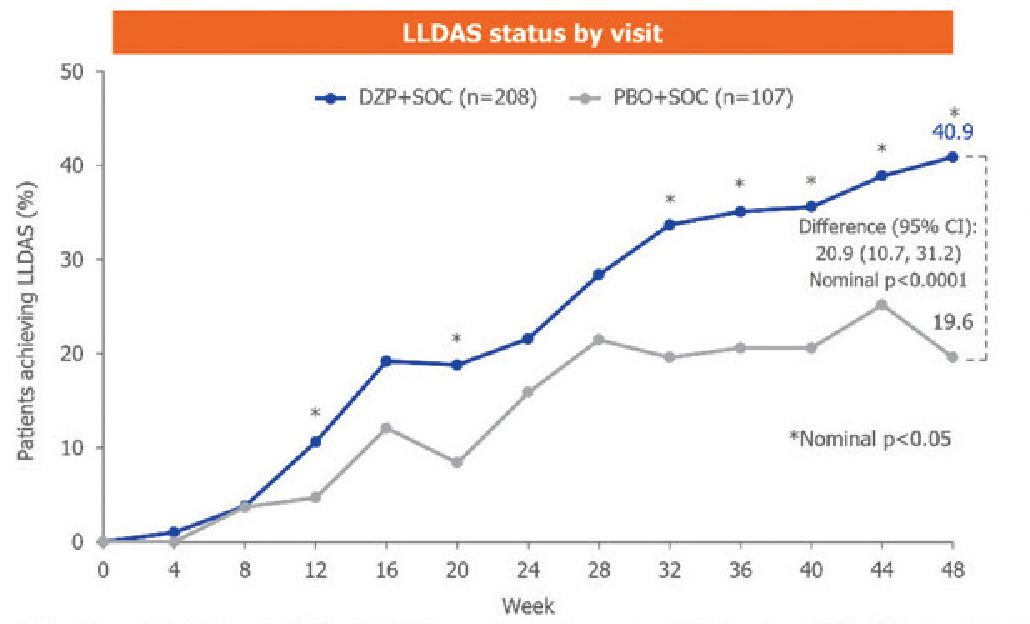
CI=confidence interval; LLDAS=Lupus Low Disease Activity State; PBO=placebo; SOC=standard of care
Figure 1. LLDAS status by visit3
PsA is a progressive, multidomain and interleukin-17 (IL-17)-linked disease that results in substantially reduced quality-of-life. Sonelokimab is a novel nanobody that binds with a similarly high affinity to IL-17A and IL-17F, inhibiting all dimers. In a phase 2 randomized controlled trial, over 60% of patients treated with sonelokimab achieved minimal disease activity within 24 weeks, with significant improvements in joint and skin symptoms.4 The small size of sonelokimab allows better tissue penetration compared with traditional monoclonal antibodies, making it a promising option for hard-to-treat inflammation.5
Janus kinase (JAK) inhibitors are an effective treatment option in RA and other autoimmune diseases. However, the use of JAK inhibitors is associated with increased levels of total cholesterol, low-density lipoprotein cholesterol, triglycerides, and creatinine kinase, reducing the net clinical benefit of using them. Adding Rho-associated protein kinase (ROCK) inhibition to JAK inhibition might provide cardioprotection as ROCK inhibitors have been shown to reduce vascular inflammation, improve endothelial function, and prevent cardiac remodelling in preclinical models.6
CPL’116 represents a new class of dual inhibitors targeting both JAK and ROCK pathways. The findings of a phase 2 randomized trial suggest a dose-dependent response with CPL'116, with significant efficacy at the high dose of 240 mg twice daily in patients with RA unresponsive to methotrexate. CPL’116 was generally well tolerated and was not associated with lipid abnormalities or creatinine kinase increase. These findings warrant further investigation in larger studies and different clinical settings.6
A revolutionary pH-sensitive gel developed by Cambridge University responds to inflammation by releasing drugs only at affected sites. This smart material detects acidity changes caused by arthritis flare-ups and delivers pain relief precisely where needed, minimizing systemic exposure and side effects.7
The Food and Drug Administration (FDA) has recently approved the SetPoint System, a neuroimmune modulation device, for the treatment of adults with moderately to severely active RA who have had an inadequate response, loss of response or intolerance to 1 or more biological or targeted synthetic disease-modifying anti-rheumatic drugs (b/tsDMARDs). In a phase 3 study, the SetPoint neuroimmune modulation device was found to be an effective treatment for patients with difficult-to-treat RA. The device is surgically placed under general anesthesia on the vagus nerve. By delivering electrical stimulation to the vagus nerve, the treatment is expected to reduce inflammation without immunosuppression and restore the immune pathways that are believed to be dysfunctional in RA patients.8
What makes the diagnosis of rheumatic disease particularly challenging is its very heterogeneous clinical presentations. The need for reliable, disease-specific biomarkers is more urgent than ever to make differential diagnosis easier and assess disease progression and severity over time.9 Biomarkers play an essential role in advancing precision medicine, offering a strategic opportunity to improve health and lower healthcare costs.10
For example, the autoimmune mechanisms driving RA often start years before noticeable symptoms like joint pain and stiffness arise. Diagnostic biomarkers are essential tools for clinicians during this phase, as they enable the accurate identification of individuals at risk or in the preclinical stages of the disease, facilitating the use of this optimal period for intervention.11 In addition to their diagnostic value, numerous RA biomarkers serve diverse functions across different stages of the disease, supporting risk assessment, monitoring disease activity, and informing treatment decisions (Table 1).2
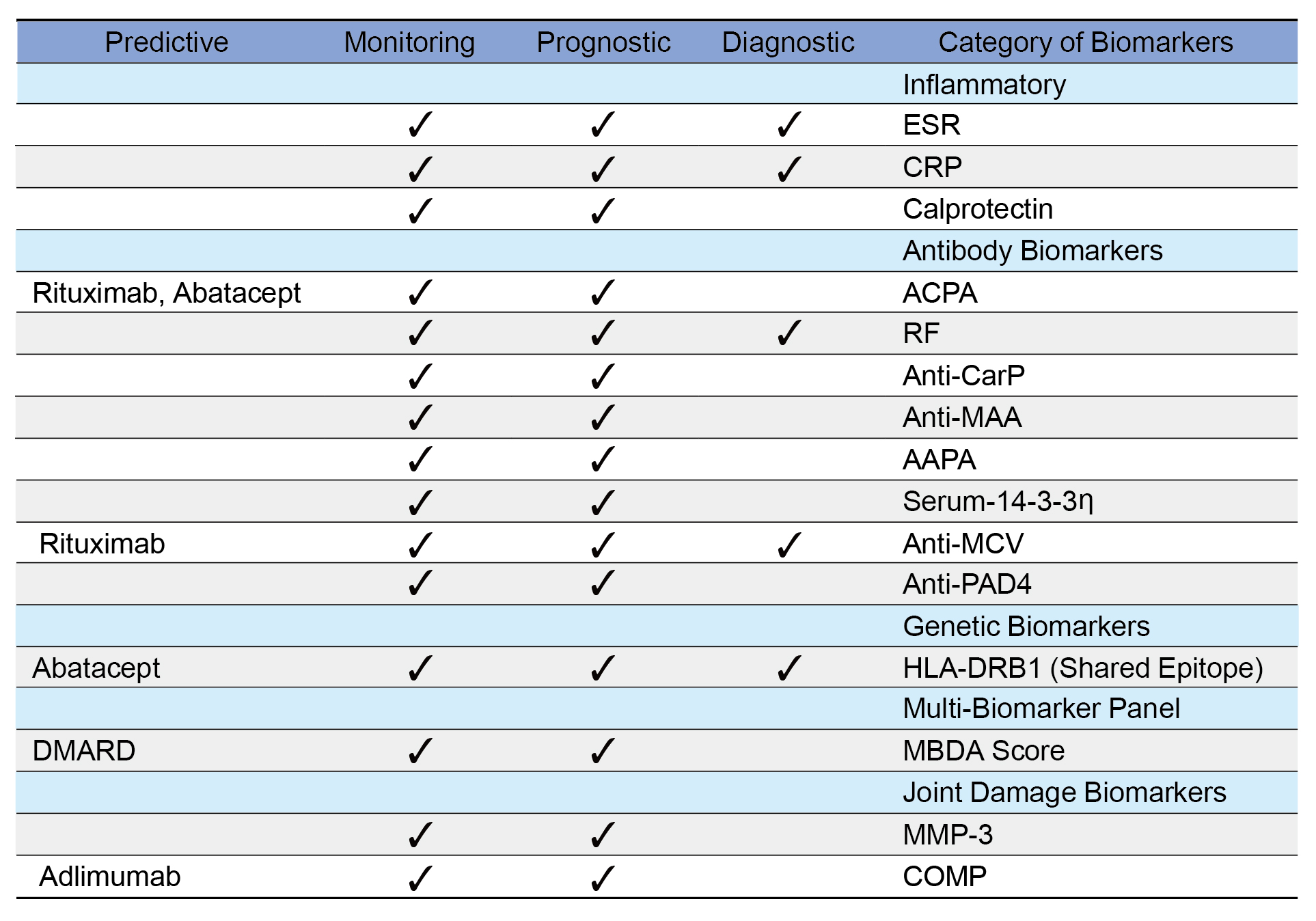
AAPA=anti-acetylated protein antibodies; ACPA=anti-citrullinated protein antibodies; anti-CarP=anti-carbamylated protein antibodies; anti-MAA=anti-malondialdehyde-acetaldehyde antibodies; anti-MCV=anti-mutated citrullinated vimentin; anti-PAD4=anti-peptidyl arginine deiminase 4; COMP=cartilage oligomeric matrix protein; CRP=C-reactive protein; DMARD—disease-modifying anti-rheumatic drug; ESR=erythrocyte sedimentation rate; HLA-DRB1=human leukocyte antigen DR beta 1; mBDA Score=multi-biomarker disease activity score; MMP-3=matrix metalloproteinase-3; RF=rheumatoid factor; Serum-14-3-3η=serum14-3-3 eta protein
Table 1. Biomarkers in RA categorized according to their diagnostic, prognostic, monitoring, and predictive roles2
The integration of AI into rheumatology has revolutionized research and clinical practice, offering transformative advancements in diagnostics, biomarker discovery, genomics, digital health technologies, and personalized medicine. AI is being used to analyze imaging, lab results, and patient-reported outcomes to assist in early diagnosis and treatment optimization. AI tools are particularly useful in distinguishing between overlapping autoimmune conditions.12, 13, 14
Telehealth has become a cornerstone of rheumatology care. Using wearable health technologies, smart devices like smart watches and smartphone sensors can objectively and continuously monitor disease activity and patient function for conditions like RA. In 2025, virtual clinics are equipped with remote monitoring tools that track joint swelling, mobility, and fatigue in real time. These data inform treatment adjustments and reduce the need for in-person visits. Apps using AI algorithms now help patients log symptoms and predict flare-ups, enabling proactive care and medication adjustments.13
In 2005, the FDA expanded the use of guselkumab to children with plaque psoriasis (PsO) and PsA, offering a safer and more effective alternative to corticosteroids. The FDA’s approval includes the treatment of pediatric patients 6 years and older weighing at least 40 kg with moderate to severe PsO, who are candidates for systemic therapy or phototherapy, and for the treatment of active PsA. Previously, these indications were only approved in adults. The approval for active PsA was supported by pharmacokinetic data extrapolated from prior PsO and PsA studies. Findings showed that the efficacy and safety data of guselkumab in pediatric patients with PsA was consistent with that from adults with PsO and PsA and children with moderate to severe PsO.15
IgG4-related disease is a multiorgan, relapsing, fibroinflammatory, immune-mediated disorder with no approved therapy before 2025. Inebilizumab targets and depletes CD19+ B cells; and according to a phase 3 trial, inebilizumab reduced the risk of flares of IgG4-related disease and increased the likelihood of flare-free complete remission at 1 year.16 In April 2025, inebilizumab became the first FDA-approved therapy for IgG4-related disease, addressing a major unmet need in this rare autoimmune condition. This approval offers a new therapeutic option for patients previously reliant on less targeted treatments.17
Despite the remarkable progress in rheumatology in 2025, several challenges continue to shape the trajectory of care and research. Addressing these issues will be critical to ensuring that innovations translate into meaningful, equitable, and sustainable improvements for all patients.
While biologics, nanobody therapies, and implantable devices offer transformative benefits, their high costs remain a barrier for many patients, particularly in low- and middle-income countries. Reimbursement policies, patent protections, and limited availability of biosimilars contribute to disparities in access. Expanding global manufacturing, streamlining regulatory pathways, and fostering public-private partnerships will be essential to make advanced therapies more affordable and widely available.
Many of the newly approved treatments lack long-term safety data, especially in diverse populations and those with comorbidities. Post-marketing surveillance, patient registries, and real-world evidence studies must be prioritized to monitor adverse effects, treatment durability, and comparative effectiveness. These data will also help refine treatment guidelines and inform shared decision-making.
Autoimmune diseases often present with overlapping symptoms and comorbid conditions, complicating diagnosis and management. Although AI-driven tools and biomarker profiling are improving diagnostic accuracy, further refinement is needed to distinguish between similar conditions and predict disease progression. Investment in multi-omics research and integrative diagnostic platforms will be key to overcoming these challenges.
The growing demand for rheumatologic care is outpacing the availability of trained specialists. In many regions, patients face long wait times and limited access to expert care. Expanding training programs, incorporating telemedicine into routine practice, and leveraging AI to support clinical decision-making can help bridge this gap and improve care delivery.
Empowering patients to actively participate in their care is vital for long-term disease management. However, disparities in digital literacy, language barriers, and limited access to technology can hinder engagement. Tailored education programs, culturally sensitive communication strategies, and inclusive digital tools must be developed to ensure that all patients benefit from the evolving landscape of rheumatology.
Looking ahead, to fully realize the potential of these advances, the rheumatology community must embrace a holistic and collaborative approach. Key priorities for the future include:
The year 2025 stands as a transformative milestone in rheumatology, marked by a convergence of scientific innovation, technological advancement, and patient-centered care. From nanobody therapies and smart drug delivery systems to AI-enhanced diagnostics and neuroimmune modulation devices, the field is rapidly evolving beyond traditional paradigms. These breakthroughs are not only enhancing disease control and reducing treatment burdens but are also reshaping how clinicians approach autoimmune and inflammatory conditions—with greater precision, personalization, and compassion.
Importantly, these innovations are empowering patients to take a more active role in their health journeys, supported by tools that offer real-time insights, remote monitoring, and tailored therapies. As the boundaries between disciplines continue to blur, collaboration among rheumatologists, immunologists, engineers, and data scientists will be essential to sustain this momentum.
Looking ahead, the challenge will be to ensure equitable access to these advances across diverse populations and healthcare systems. Continued investment in research, education, and global partnerships will be critical to translating these breakthroughs into meaningful, lasting improvements in quality of life for all individuals affected by rheumatic diseases.
In embracing this new era, the rheumatology community reaffirms its commitment to alleviating suffering, restoring function, and advancing care—guided by innovation, grounded in evidence, and inspired by the resilience of its patients.
References
1. Mayo Clinic. Rheumatology. 1 April 2020. Available from: https://www.mayoclinic.org/departments-centers/rheumatology/sections/overview/ovc-20477132. [Accessed 6 October 2025]. 2. Colina M and Campana G. J Clin Med. 2025;14(5):1735. 3. Morand E, et al. Annals of the Rheumatic Diseases. 2025; 84(Suppl.1):167–8. 4. McInnes IB, et al. Nat Med. 2025 Oct 6. doi: 10.1038/s41591-025-03971-6. Online ahead of print. 5. Li Z, et al. MAbs. 2016;8(1):113–9. 6. Wieczorek M, et al. Lancet Rheumatol. 2025;7(9):e629–e641. 7. University of Cambridge. Cambridge scientists created a gel that could end arthritis pain. ScienceDaily, 25 September 2025. Available from: www.sciencedaily.com/releases/2025/09/250925025401.htm. [Accessed 6 October 2025]. 8. Rheumatology Advisor. FDA Approves Neuroimmune Modulation Device for Rheumatoid Arthritis. 6 August 2025. Available from: https://www.rheumatologyadvisor.com/news/fda-approves-neuroimmune-modulation-device-for-rheumatoid-arthritis/. [Accessed 8 October 2025]. 9. Mohan C and Assassi S. BMJ. 2015;351:h5079. 10. Lin CMA, et al. Nat Rev Rheumatol. 2022; 18: 725–33. 11. Guo Q, et al. Bone Res. 2018; 6:15. 12. Sequí-Sabater JM, et al. RMD Open. 2025 Jan 8;11(1):e004309. 13. Al Zo'ubi M. Clin Rheumatol. 2025;44(4):1427–38. 14. Liu S, et al. Front Immunol. 2025;16:1525462. 15. Rheumatology Advisor. Tremfya Approved for Children With Plaque Psoriasis, Psoriatic Arthritis. 6 October 2025. Available from: https://www.rheumatologyadvisor.com/news/tremfya-approved-for-children-with-plaque-psoriasis-psoriatic-arthritis/. [Accessed 8 October 2025]. 16. Stone JH, et al. N Engl J Med. 2025;392(12):1168–77. 17. HCP Live. Rheumatology Month in Review: April 2025. 3 April 2025. Available from: https://www.hcplive.com/view/rheumatology-month-in-review-april-2025. [6 October 2025]. 18. Caffarel-Salvador E, et al. Curr Opin Pharmacol. 2017;36:8–13. 19. Tsitrouli Z, et al. BioTech (Basel). 2021;10(3):11.